o-Carborane
Synonym(s):1,2-Dicarbadodecaborane(12)
- CAS NO.:16872-09-6
- Empirical Formula: C2B10
- Molecular Weight: 132.12
- MDL number: MFCD00012169
- EINECS: 240-897-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
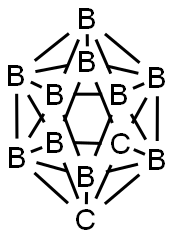
What is o-Carborane?
Description
Boranes are boron–hydrogen compounds, most of which contain delocalized electron bonds. Boranes with four or more boron atoms typically have completely or partially closed polyhedral cages. Examples are former Molecules of the Week tetraborane, pentaborane, decaborane, and cesium dodecaborate.
Similarly, carboranes are complete (closed) or partial borane polyhedra in which one or more of the boron atoms are replaced by carbon atoms. Carboranes have a nomenclature of their own: [n1,n2,n3, . . .][structural prefix][carbon prefix]carba[total atom prefix]borane, in which
As an example, 2,3-nido-dicarbahexaborane is one atom short of a polyhedron with two carbon atoms in the 2- and 3-positions of a six-atom structure.
Of particular interest are the closo-dicarbadodecaboranes, which have two carbon atoms in complete 12-atom icosahedral structures. Of the three possible isomers, 1,2-dicarbadodecaborane is shown here. The other isomers have carbon atoms in the 1,7- and 1,12-positions. In some publications, the 1,2-, 1,7-, and 1,12-prefixes are denoted as o- (ortho-), m- (meta-), and p- (para-), respectively.
Unlike many boranes and carboranes, the closo-dicarbadodecaboranes are extremely stable. They are crystalline solids with high melting points and low solubility in water and organic solvents. They are somewhat interchangeable in that heating the 1,2-isomer to 420° C rearranges it to the 1,7-species, which upon additional heating to >600 °C rearranges to the 1,12-isomer.
1,2-Dicarbadodecaborane is prepared by the reaction of open-cage decaborane with acetylene, usually by first “priming” the decaborane with an adduct such as diethyl sulfide before the reaction with acetylene. 1,2-Dicarbadodecaborane derivatives are similarly synthesized by treating decaborane with substituted acetylenes.
Chemically, closo-dicarbadodecaboranes react similarly to organic cyclic compounds in that hydrogen atoms on carbon or boron vertices can be replaced by a wide range of substituents. For example, in a 1995 study, Peggy A. Radel and Stephen B. Kahl* at the University of California, San Francisco, used a several-step process to synthesize L- and D-enantiomers of 1,2-dicarbadodecaborane-substituted alanines. The alanine methyl groups were bonded to one of the carbon atoms on the dicarbadodecaborane cage.
Earlier this year, Alexander M. Genaev and co-workers at the N. N. Vorozhtsov Institute of Organic Chemistry (Novosibirsk, Russia) reported the noncatalyzed bromination of 1,2-dicarbadodecaborane at the 8- and 9-boron atoms on the icosahedral structure.
Chemical properties
WHITE TO SLIGHTLY GREYISH CRYST. POWDER OR CHUNKS
The Uses of o-Carborane
o-Carborane is used in a wide range of applications such as heat-resistant polymers and medical applications. In coordination chemistry, it is used as unique bulky ligand scaffolds.
What are the applications of Application
o-Carborane is a boron-rich cluster
Properties of o-Carborane
| Melting point: | 285-287°C |
| Density | 0.95 |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Chloroform (Sparingly), DMSO (Slightly), Methanol (Slightly) |
| appearance | white crystals or powder |
| form | Powder |
| color | White |
| Water Solubility | Insoluble in water. |
| NIST Chemistry Reference | 1,2-Dicarbadodecaborane(12)(16872-09-6) |
| EPA Substance Registry System | 1,2-Dicarbadodecaborane(12) (16872-09-6) |
Safety information for o-Carborane
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for o-Carborane
| InChIKey | UFWBKRZLYJDOMC-UHFFFAOYSA-N |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




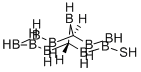
You may like
-
 o-Carborane 95% CAS 16872-09-6View Details
o-Carborane 95% CAS 16872-09-6View Details
16872-09-6 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1