Nosiheptide
- CAS NO.:56377-79-8
- Empirical Formula: C51H43N13O12S6
- Molecular Weight: 1222.34
- MDL number: MFCD30718117
- EINECS: 260-138-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
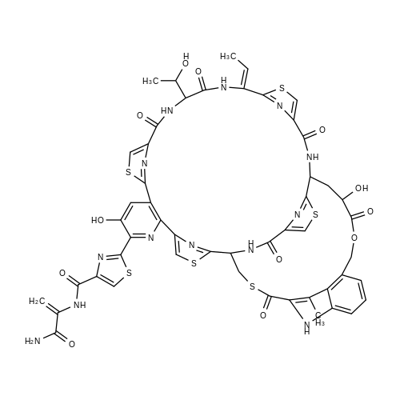
What is Nosiheptide?
The Uses of Nosiheptide
Nosiheptide is an antibacterial item used in feedstock and feed additives for chickens.
The Uses of Nosiheptide
Nosiheptide is a bicyclic thiopeptide antibiotic produced by several species of actinomycetes, notably Streptomyces, first reported by Japanese researchers in 1970. Unlike other bicyclic thiopeptides such as thiostrepton, the second macrocyclic ring is linked by relatively fragile lactone and thiolactone bridges to the core cyclic peptide. Nosiheptide has broad spectrum antibacterial activity, and has recently demonstrated a prolonged post-antibiotic effect in both nosocomial and community-acquired MRSA compared with vancomycin. Despite its long history in animal health, nosiheptide has not been extensively studied and is regarded as a “lost antibiotic” largely escaping intensive investigation for human application.
What are the applications of Application
Nosiheptide is a bicyclic thiopeptide antibiotic
Biological Activity
mic: ≤ 0.25 mg/l for methicillin-resistant s. aureus strains; ≤ 0.125 mg/l for enterococcus spp; 0.008 mg/l for bi strain of c. difficilenosiheptide is a thiopeptide antibiotic.thiopeptides are a family of antibiotics counting with more than one hundred different entities. although thiopeptides are mainly isolated from soil bacteria, new members have been isolated from marine samples. thiopeptides have been reported to have a wide range of biological properties, such as antiplasmodial, anticancer, immunosuppressive, etc.
in vitro
the mode of action of nosiheptide on bacterial protein synthesis was found to be closely similar to that of thiostrepton. both antibiotics could inhibit functions of elongation factors tu and g and could also significantly reduce the synthesis of guanosine penta- and tetraphosphates to stringent factor. in addition, the actinomycetes that produced nosiheptide were able to defend themselves against their products in similar fashion, which involved specific pentosemethylation of 23s ribosomal rna [1].
in vivo
the rhdl–nosiheptide complex was intravenously administered to male wistar rats and the distribution of nosiheptide in the liver and plasma was monitored 30 min after injection. results showed that the hepatic distribution of the rhdl–nosiheptide complex accounted for most of the administered nosiheptide, and was seven times as much as that in plasma. these findings indicated that the rhdl–nosiheptide complex could targete nosiheptide to the liver [2].
References
[1] e. cundliffe and j. thompson. the mode of action of nosiheptide (multhiomycin) and the mechanism of resistance in the producing organism. j.gen.microbiol. 126(1), 185-192 (1981).
[2] feng m, cai q, shi x, huang h, zhou p, guo x. recombinant high-density lipoprotein complex as a targeting system of nosiheptide to liver cells. j drug target. 2008 jul;16(6):502-8.
Properties of Nosiheptide
| Melting point: | 310-320° (dec) |
| alpha | D20 +38° (c = 1 in pyridine) |
| Density | 1.534±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 1.42±0.60(Predicted) |
| color | Light Yellow to Light Brown |
Safety information for Nosiheptide
Computed Descriptors for Nosiheptide
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4