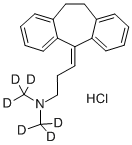
NORTRIPTYLINE HYDROCHLORIDE
Synonym(s):3-(10,11-Dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N-methyl-1-propanamine hydrochloride;Desmethylamitriptyline hydrochloride;Nortriptyline hydrochloride
- CAS NO.:894-71-3
- Empirical Formula: C19H22ClN
- Molecular Weight: 299.84
- MDL number: MFCD00058024
- EINECS: 212-973-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04

What is NORTRIPTYLINE HYDROCHLORIDE?
Chemical properties
Off-White Solid
Originator
Aventyl,Lilly,UK,1963
The Uses of NORTRIPTYLINE HYDROCHLORIDE
Nortriptyline Hydrochloride (Amitriptyline EP Impurity C) is a tricyclic antidepressant.
The Uses of NORTRIPTYLINE HYDROCHLORIDE
Monoamine reuptake inhibitor; tricyclic antidepressant.
What are the applications of Application
Nortriptyline Hydrochloride is a 5-HT2 serotonin receptor antagonist
Definition
ChEBI: Nortriptyline hydrochloride is an organic tricyclic compound. It has a role as a geroprotector.
Manufacturing Process
A mixture of 114.5 g of 5-(3-chloropropylidene)dibenzo[a,d]cyclohepta[1,4] diene, 75 ml of benzene, and about 400 ml of methylamine is heated in an autoclave at 120°C for six hours. The excess methylamine is distilled from the reaction mixture under vacuum and the residue is stirred with 300 ml of water. Acidification of the mixture with hydrochloric acid causes the separation of the hydrochloride of 5-(3-methylaminopropylidene)dibenzo[a,d] cyclohepta[1,4]diene. The product is collected by filtration and is purified by recrystallization from a mixture of absolute ethanol and ethyl acetate. MP 210°C to 212°C.
brand name
Aventyl Hydrochloride (Lilly); Aventyl Hydrochloride (Ranbaxy); Pamelor (Tyco).
Therapeutic Function
Antidepressant
General Description
Pertinent biological and chemical properties for nortriptyline,3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)Nmethyl-1-propanamine hydrochloride, 5-(3-methyl-aminopropylidene)-10,11-hydro-5H-dibenzo[a,d] cycloheptene hydrochloride(Aventyl, Pamelor), are given previously in thediscussion of amitriptyline. Metabolic inactivation and eliminationare like those of amitriptyline. Nortriptyline is a selectiveNE transporter (NET) inhibitor.
Biological Activity
ki: 3.4 nm: blocks the norepinephrine transporter.ki: 161 nm: inhibits the serotonin transporter.nortriptyline, a dibenzocycloheptene-derivative tricyclic antidepressant, inhibits norepinephrine and serotonin transporters, which is used for short-term treatment of various forms of depression, including major depression, dysthymia, and atypical depressions. nortriptyline suppresses the norepinephrine presynaptic receptors, thus inhibiting the reuptake of this neurotransmitter and increasing the concentration in the synaptic cleft in the central nervous system.norepinephrine transporter is responsible for the sodium-chloride (na+/cl-) -dependent reuptake of extracellular norepinephrine. serotonin transporter transports serotonin from the synaptic cleft to the presynaptic neuron.
Biochem/physiol Actions
Tricyclic antidepressant that is a more potent inhibitor of the norepinephrine transporter (Ki = 3.4 nM) than the serotonin transporter (Ki = 161 nM). 5-HT2 serotonin receptor antagonist.
in vitro
nortriptyline dose-dependently dampened the spontaneous migration of human polymorphonuclear cells (pmn) at the highest concentrations (10-4 and 10-5 m). nortriptyline decreased the motility of the cells at 10-4m. chemotaxis of pmn triggered by 10-10m n-formyl-l-methionil-l-leucyl-l-phenylalanine (fmlp) was blocked by nortriptyline in a dose-dependent fashion [1].
in vivo
rats and mice were injected intraperitoneally with nortriptyline at the dose of 50, 25, 12.5 mg/kg. after 2 hours of the last injection, nortriptyline inducted little or no inhibition of the 4, α-dimethyl-meta-tyramine (h 77/77) induced depletion in a dose of 25 + 12.5 mg/kg. however, there was a partial blockade, evoked by nortriptyline in a dose of 50 + 25 mg/kg, of the h 77/77 elicited depletion in the noradrenaline nerve terminals in all the rats studied. additionally, nortriptyline also proved to be active in the brain, however, its activity was low [2].
References
[1]. sacerdote, p., bianchi, m., & panerai, a. chlorimipramine and nortriptyline but not fluoxetine and fluvoxamine inhibit human polymorphonuclear cell chemotaxis in vitro. general pharmacology: the vascular system. 1994; 25(3): 409-412.
[2]. carlsson, a., corrodi, h., fuxe, k., & hkfelt, t. effects of some antidepressant drugs on the depletion of intraneuronal brain catecholamine stores caused by 4, α-dimethyl-meta-tyramine. european journal of pharmacology.1969; 5(4): 367-373.
Properties of NORTRIPTYLINE HYDROCHLORIDE
| Melting point: | 217-220?C |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | ethanol: 100 mg/mL |
| form | powder |
| color | white to off-white |
| λmax | 237nm(H2O)(lit.) |
| Merck | 14,6715 |
Safety information for NORTRIPTYLINE HYDROCHLORIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for NORTRIPTYLINE HYDROCHLORIDE
NORTRIPTYLINE HYDROCHLORIDE manufacturer
Festiva Pharma
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
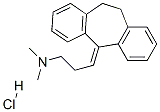



You may like
-
 Nortriptyline Hydrochloride >95% CAS 894-71-3View Details
Nortriptyline Hydrochloride >95% CAS 894-71-3View Details
894-71-3 -
 Nortriptyline Hydrochloride CAS 894-71-3View Details
Nortriptyline Hydrochloride CAS 894-71-3View Details
894-71-3 -
 Nortriptyline hydrochloride CAS 894-71-3View Details
Nortriptyline hydrochloride CAS 894-71-3View Details
894-71-3 -
 Nortriptyline hydrochloride CAS 894-71-3View Details
Nortriptyline hydrochloride CAS 894-71-3View Details
894-71-3 -
 Nortriptyline Hydrochloride CAS 894-71-3View Details
Nortriptyline Hydrochloride CAS 894-71-3View Details
894-71-3 -
 Nortriptyline hydrochloride CAS 894-71-3View Details
Nortriptyline hydrochloride CAS 894-71-3View Details
894-71-3 -
 Nortriptyline hydrochloride CAS 894-71-3View Details
Nortriptyline hydrochloride CAS 894-71-3View Details
894-71-3 -
 Nortriptyline hydrochloride solution CAS 894-71-3View Details
Nortriptyline hydrochloride solution CAS 894-71-3View Details
894-71-3
