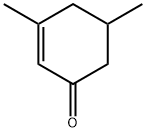
NOOTKATONE
Synonym(s):(+)-Nootkatone
- CAS NO.:4674-50-4
- Empirical Formula: C15H22O
- Molecular Weight: 218.33
- MDL number: MFCD00036591
- EINECS: 225-124-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-25 18:09:02
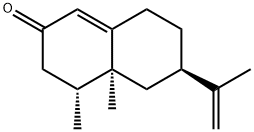
What is NOOTKATONE?
Description
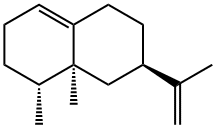
Nootkatone has a pleasant taste. It may be prepared by oxidation of valencene (a sesquiterpene) with tertiary butyl chromate.
Description
(+)-Nootkatone is sesquiterpene ketone originally isolated from grapefruit juice and peel oil with diverse biological activity. It is lethal against ticks, fleas, and mosquitoes with LC50 values of 0.0029, 0.0061, and 0.0046% w/v, respectively. Pretreatment of wood with (+)-nootkatone reduces tunnel lengths, feeding, and survival rates in C. formosanus termites. (+)-Nootkatone (10-100 μM) dose-dependently activates AMPKα1 and AMPKα2 in C2C12 mouse myoblast lysate containing a substrate peptide. It dose-dependently inhibits platelet aggregation induced by collagen, thrombin , and arachidonic acid when used at concentrations ranging from 10 to 100 μM with almost complete inhibition at the highest concentration. In vivo, (+)-nootkatone (3-30 mg/kg, p.o.) dose-dependently increases the length of tail bleeding time in mice. (+)-Nootkatone (0.1-0.3%, p.o.) dose-dependently reduces body weight and plasma glucose levels in mice fed a high-fat and high-sucrose diet.
Chemical properties
viscous yellow liquid or crystals with a citrus odour;
Chemical properties
NOOTKATONE has been isolated from grapefruit peel and juice
and identified in other citrus oils as well. The commercially available product is a
colorless to yellowish liquid with a typical grapefruit odor.
Nootkatone can be prepared by oxidation of valencene, a sesquiterpene hydrocarbon
isolated from orange oils. The oxidation can be accomplished either by
chemical or by biotechnological methods.
Nootkatone is used for flavoring beverages.
Chemical properties
Nootkatone has a powerful fruity sweet, citrusy, grapefruit peel oil-like aroma.
Occurrence
Reported found in grapefruit oil and juice; traces are also reported found in the oils of bergamot, lemon, lime, orange and tangerine; also reported formed in canned orange juice on storage.
The Uses of NOOTKATONE
Nootkatone is an effective repellent/insecticide against mosquitos, and may repel bed bugs, head lice and other insects. Nootkatone in spray form has been shown as an effective repellent/insecticide against deer ticks and lone star ticks.
Definition
ChEBI: A sesquiterpenoid that is 4,4a,5,6,7,8-hexahydronaphthalen-2(3H)-one which is substituted by methyl groups at positions 4 and 4a, and by an isopropenyl group at position 6 (the 4R,4aS,6R stereoisomer).
Preparation
By oxidation of valencene (a sesquiterpene) with tertiary butyl chromate.
Aroma threshold values
Detection: 170 to 800 ppb
Taste threshold values
Taste characteristics at 20 ppm: grapefruit, citrus, orange and butter.
General Description
(+)-nootkatone, a bicyclic conjugated sesquiterpene ketone with a grapefruit-like flavor, is commonly used in fragrance, food, cosmetics and pharmaceutical industry. It can be synthesized from (+)-valencene via biotransformation. (+)-nootkatone is the active ingredient responsible for the antiplatelet effect of Cyperus rotundus, a well-known oriental traditional medicine. It also shows promising efficacy against Staphylococcus aureus biofilms.
Properties of NOOTKATONE
| Melting point: | 35-39 °C |
| Boiling point: | 125°C 0,5mm |
| Density | 0,997 g/cm3 |
| refractive index | n |
| FEMA | 3166 | NOOTKATONE |
| Flash point: | 99°C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Ethanol (Slightly), Methanol (Slightly) |
| form | neat |
| form | Solid |
| color | White to Pale Yellow Low-Melting |
| Odor | at 100.00 %. grapefruit peel citrus gardenia woody |
| optical activity | [α]20/D +182.0±5.0°, c = 1% in ethanol |
| Water Solubility | Slightly soluble in ethanol and chloroform. Partly soluble in water. |
| JECFA Number | 1398 |
| BRN | 4676969 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| EPA Substance Registry System | 2(3H)-Naphthalenone, 4,4a,5,6,7,8-hexahydro-4,4a-dimethyl-6-(1-methylethenyl)-, (4R,4aS,6R)- (4674-50-4) |
Safety information for NOOTKATONE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for NOOTKATONE
| InChIKey | WTOYNNBCKUYIKC-UHFFFAOYSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 6-Bromo-3-iodo-1-methyl-1H-indazole 4-Ethylbenzylamine N-(5-Amino-2-methylphenyl)acetamide 2-(BOC-Amino)4-picoline 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazoleRelated products of tetrahydrofuran




You may like
-
 Nootkatone, ≥98% CAS 4674-50-4View Details
Nootkatone, ≥98% CAS 4674-50-4View Details
4674-50-4 -
 (+)-Nootkatone CAS 4674-50-4View Details
(+)-Nootkatone CAS 4674-50-4View Details
4674-50-4 -
 (+)-Nootkatone CAS 4674-50-4View Details
(+)-Nootkatone CAS 4674-50-4View Details
4674-50-4 -
 100-71-0 99%View Details
100-71-0 99%View Details
100-71-0 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 127464-43-1 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 127464-43-1 99%View Details
127464-43-1 -
 13162-05-5 N-Vinylformamide 99%View Details
13162-05-5 N-Vinylformamide 99%View Details
13162-05-5 -
 1446013-08-6 98%View Details
1446013-08-6 98%View Details
1446013-08-6 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3