NOMIFENSINE MALEATE
- CAS NO.:24526-64-5
- Empirical Formula: C16H18N2
- Molecular Weight: 238.33
- MDL number: MFCD00242716
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
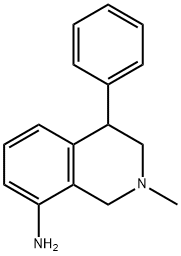
What is NOMIFENSINE MALEATE?
Toxicity
A likely cause of nomifensine toxicity is the aromatic amine group, as compounds containing this chemical substructure are notorious for producing toxic metabolites.
Description
Nomifensine is an inhibitor of norepinephrine (NE) and dopamine (DA) reuptake. It inhibits uptake of NE, DA, and serotonin (5-HT) in rat brain synaptosomes with IC50 values of 6.6, 48, and 830 nM, respectively. It is selective for DA, NE, and 5-HT uptake inhibition over binding to dopamine D2, α1- adrenergic-, 5-HT2, and muscarinic receptors (IC50s = 43,000, 1,200, 3,800, and >13,000 nM, respectively, in rat brain membranes). Nomifensine is selective for inhibition of NE over DA uptake in vivo with minimal inhibitory doses of 28 and less than 57 μmol/kg, respectively. It decreases the time Wistar Kyoto, but not Sprague-Dawley, rats spend immobile in the forced swim test but also increases locomotor activity in the open field test in Wistar Kyoto and Sprague-Dawley rats when administered at a chronic dose of 10 mg/kg.
Originator
Alival,Hoechst,W. Germany ,1976
The Uses of NOMIFENSINE MALEATE
A novel antidepressant distinguished from existing tricyclic and tetracyclic antidepressants by its bicyclic structure.
Background
Nomifensine, formerly marketed as Merital capsules, was associated with an increased incidence of hemolytic anemia. The approved application holder removed Merital capsules from the market on January 23, 1986. FDA published a notice of its determination that Merital capsules were removed from the market for safety reasons (see the Federal Register of June 17, 1986 (51 FR 21981)). Approval of the NDA for Merital capsules was withdrawn on March 20, 1992 (see the Federal Register of March 20, 1992 (57 FR 9729)). Also withdrawn from the Canadian and UK markets.
What are the applications of Application
Nomifensine is a norepinephrine-dopamine reuptake inhibitor
Definition
ChEBI: An N-methylated tetrahydroisoquinoline carrying phenyl and amino substituents at positions C-4 and C-8, respectively.
Manufacturing Process
A solution of N-(2-aminobenzyl)-1-phenyl-2-methylaminoethanol-1 was prepared by the reaction of α-bromo-acetophenone and (2nitrobenzyl)methylamine, followed by hydrogenation of the nitro group by means of nickel on diatomaceous earth at room temperature and reduction of the CO group by means of sodium borohydride. The intermediate thus produced was dissolved in 100 ml of methylene chloride and introduced dropwise into 125 ml of sulfuric acid at 10° to 15°C. After a short standing, the reaction mixture was poured onto ice and rendered alkaline by means of a sodium hydroxide solution. By extraction with ether, there was obtained 1,2,3,4-tetrahydro-2-methyl-4-phenyl-8-amino-isoquinoline. The base is reacted with maleic acid to give the maleate; melting point of the maleate 199° to 201°C (from ethanol).
brand name
Merital (Hoechst-Roussel);Anametrin;Caribium;Hoe 984;Hostalival;Merival;Musettamycin;Neurolene;Nomival;Psicronizer;Psyton.
Therapeutic Function
Psychostimulant
World Health Organization (WHO)
Nomifensine, an antidepressant indicated for the treatment of a wide range of depressive illness, was introduced in 1976. Subsequently rare cases of haemolytic anaemia - sometimes fatal - thrombocytopenia, hepatotoxicity and fever were associated with the use of the drug. Following discussions with regulatory authorities in the United Kingdom and the Federal Republic of Germany the major manufacturer withdrew all preparations containing nomifensine worldwide in January 1986.
Pharmacokinetics
Nomifensine is a dopamine reuptake inhibitor test-marketed in the United States by Hoechst AG (now Novartis) that increases the amount of synaptic dopamine available to receptors by blocking dopamine's re-uptake transporter. Nomifensine is now mainly used in scientific research, particularly in studies involving dopamine release in response to addiction.
Safety Profile
Poison by ingestion andintravenous routes. Human systemic effects by ingestion:diffuse hepatitis, hemorrhage and decrease in the numberof blood platelets (thrombocytopenia). When heated todecomposition it emits toxic fumes of NOx.
Metabolism
Not Available
Properties of NOMIFENSINE MALEATE
| Melting point: | 179-181° |
| Boiling point: | 370.93°C (rough estimate) |
| Density | 0.9597 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| storage temp. | 2-8°C(protect from light) |
| solubility | DMF: 25 mg/ml; DMSO: 25 mg/ml; Ethanol: 10 mg/ml; PBS (pH 7.2): Partially soluble |
| form | A crystalline solid |
| pka | 7.85±0.40(Predicted) |
| color | Light yellow to yellow |
| EPA Substance Registry System | Nomifensine (24526-64-5) |
Safety information for NOMIFENSINE MALEATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for NOMIFENSINE MALEATE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
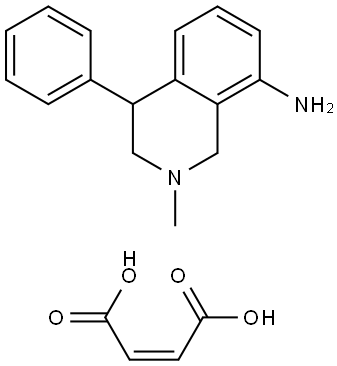

You may like
-
 Nomifensine 95% CAS 24526-64-5View Details
Nomifensine 95% CAS 24526-64-5View Details
24526-64-5 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4