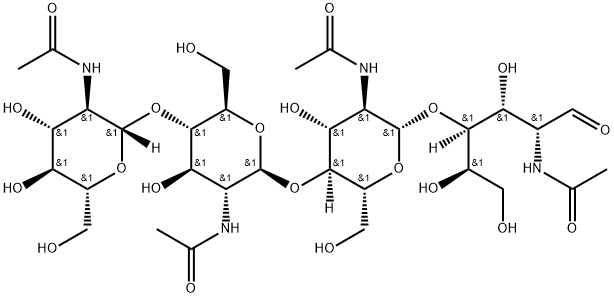
N,N',N'',N''',N'''',N'''''-Hexaacetylchitohexaose
- CAS NO.:38854-46-5
- Empirical Formula: C48H80N6O31
- Molecular Weight: 1237.17
- MDL number: MFCD00210241
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
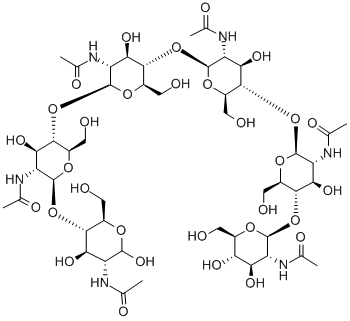
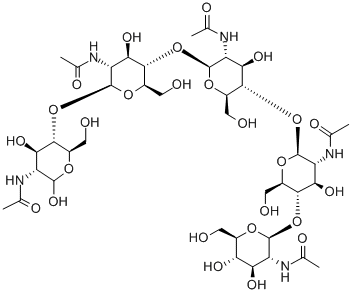
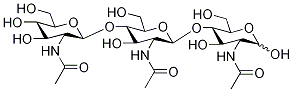
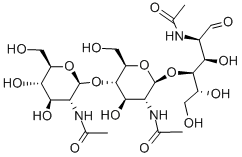
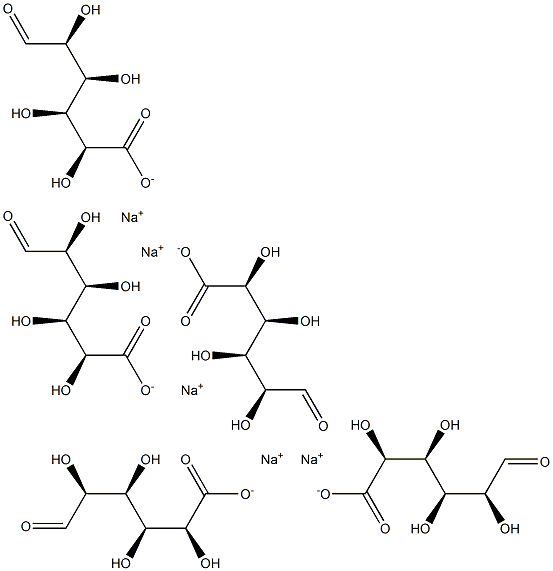
What is N,N',N'',N''',N'''',N'''''-Hexaacetylchitohexaose?
Chemical properties
White Solid
The Uses of N,N',N'',N''',N'''',N'''''-Hexaacetylchitohexaose
cation exchanger ~1.4 meq/g
The Uses of N,N',N'',N''',N'''',N'''''-Hexaacetylchitohexaose
Studied for anti-tumor effects
The Uses of N,N',N'',N''',N'''',N'''''-Hexaacetylchitohexaose
Various publications have cited the use of N,N',N'',N''',N'''',N'''''-Hexaacetylchitohexaose in mutagenesis studies due to its antitumor effects.
Hexa-N-acetylchitohexaose is a hexamer of N-acetylglucosamine, a subunit of the natural polymer chitin. It functions as an elicitor in plants, inducing the expression of chitinases.Like chitin and some of its derivatives, hexa-N-acetylchitohexaose is a substrate of lysozyme.It also binds LysM domains on certain proteins, including an endopeptidase of T. thermophilus.Hexa-N-acetylchitohexaose heightens the immune response against Pseudomonas and Listeria in mice, stimulates cytokine secretion in mesenchymal stem cells, and inhibits nitric oxide production by activated macrophages.
What are the applications of Application
N,N′,N′′,N′′′,N′′′′,N′′′′′-Hexaacetylchitohexaose is a substrate for human lysozyme, useful for mutagenesis studies.
Reactions
Chitohexaose has attracted wide interest due to its special bioactivities and these potential activities are significantly related to N-acetylation.
Herein, six chitohexaose fractions with different degrees of acetylation were prepared by selective N-acetylation and ion-exchange chromatography and further analyzed by ESI/MS.
It is revealed that all the six N-acetylated chitohexaoses were of single molecular weight, the molecular weights of which were exactly assigned to 1026.44 Da, 1068.44 Da, 1110.48 Da, 1152.48 Da, 1194.49 Da, and 1236.48 Da, respectively. These results suggested that the six prepared N-acetylated chitohexaoses were N-acetylchitohexaose (D5A1), di-N-acetylchitohexaose (D4A2), tri-N-acetylchitohexaose (D3A3), tetra-N-acetylchitohexaose (D2A4), penta-N-acetylchitohexaose (D1A5), and hexa-N-acetylchitohexaose (A6), respectively, which are of great significance to screen their bioactivities and discover well-defined chitooligosaccharide molecules as potential drugs.
- Access to N-acetylated chitohexaose with well-defined degrees of acetylation
- Li, Kecheng; Xing, Ronge; Liu, Song; Qin, Yukun; Li, Pengcheng - BioMed Research International, 2017, vol. 2017
Properties of N,N',N'',N''',N'''',N'''''-Hexaacetylchitohexaose
| Melting point: | >250°C (dec.) |
| Boiling point: | 1652.6±65.0 °C(Predicted) |
| alpha | -11.4 º (in H2O) |
| Density | 1.60±0.1 g/cm3(Predicted) |
| storage temp. | −20°C |
| solubility | Water (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 12.72±0.70(Predicted) |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for N,N',N'',N''',N'''',N'''''-Hexaacetylchitohexaose
Computed Descriptors for N,N',N'',N''',N'''',N'''''-Hexaacetylchitohexaose
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8