N',N'',N''',N''''-Pentaacetylchitopentaose
- CAS NO.:36467-68-2
- Empirical Formula: C40H67N5O26
- Molecular Weight: 1033.98
- MDL number: MFCD00210240
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:43:30
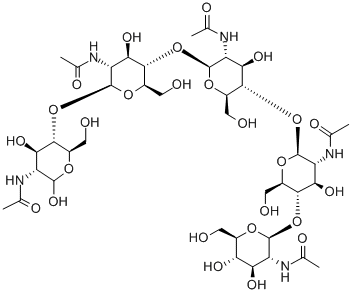
What is N',N'',N''',N''''-Pentaacetylchitopentaose?
Chemical properties
White Solid
The Uses of N',N'',N''',N''''-Pentaacetylchitopentaose
Studies have shown this compound to have anti tumor effects.
The Uses of N',N'',N''',N''''-Pentaacetylchitopentaose
- Elicits plant defense systems.
- carbohydrate component of lipo-chitooligosaccharides secreted by Rhizobia, functioning as a signaling molecule in the host plant.
- substrate for Rhizobium leguminosarum nodulation protein NodL.
What are the applications of Application
Penta-N-acetylchitopentaose is a carbohydrate component of lipo-chitooligosaccharides
Production Methods
Cell density cultivation of recombinant Escherichia coli strains harboring the nodC gene (encoding chitooligosaccharide synthase) from Azorhizobium caulinodans has been previously described as a practical method for the preparation of gram-scale quantities of penta-N-acetyl-chitopentaose. We have now extended this method to the production of allylated derivative of penta-N-acetyl-chitopentaose by using allyl 2-acetamido-2-deoxy-β-d- glucopyranoside (2) as the initial acceptor for the synthesis of target pentaoside in vivo.
- Chemo-enzymatic synthesis of allyl penta-N-acetyl-chitopentaose
- Huang, Gang-Liang; Zhang, Da-Wei; Zhao, Hong-Juan; Zhang, Hou-Cheng; Wang, Peng-George - Bioorganic and Medicinal Chemistry Letters, 2006, vol. 16, # 7, p. 2042 - 2043
Properties of N',N'',N''',N''''-Pentaacetylchitopentaose
| Melting point: | 285-295°C (dec.) |
| storage temp. | −20°C |
| solubility | PBS (pH 7.2): 1 mg/ml |
| form | powder to crystal |
| color | White to Almost white |
| Stability: | Hygroscopic |
Safety information for N',N'',N''',N''''-Pentaacetylchitopentaose
Computed Descriptors for N',N'',N''',N''''-Pentaacetylchitopentaose
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 N,N',N'',N''',N''''-Pentaacetylchitopentaose CAS 36467-68-2View Details
N,N',N'',N''',N''''-Pentaacetylchitopentaose CAS 36467-68-2View Details
36467-68-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.