NITROUS ACID
- CAS NO.:7782-77-6
- Empirical Formula: HNO2
- Molecular Weight: 47.01
- MDL number: MFCD01310438
- EINECS: 231-963-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
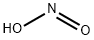
What is NITROUS ACID?
Toxicity
Oral LD50 of 157.9mg/kg observed in rats and 175mg/kg observed in mice . Estimated oral LD50 of 35mg/kg in humans . Sodium nitrite toxicity manifests as cardiovascular collapse following severe hypotension due to nitrite's vasodilatory action.
Description
Nitrous acid (molecular formula?HNO2) is a weak
and monobasic acid known only in solution and in the
form of nitrite salts. Nitrous acid rapidly decomposes
into nitrogen oxide, nitric oxide and water when in
solution:
2HNO2 ? NO2 + NO+H2O
It can also decompose into nitric acid and nitrous
oxide and water.
4HNO2 ? 2HNO3 +N2O +H2O
Nitric acid (HNO3), also known as “aqua fortis”
and “spirit of nitre”, is a highly corrosive and toxic
strong acid that can cause severe burns. It is colorless
when pure and a slight yellow when “glacial”. Older
samples tend to acquire a yellow cast due to the accumulation
of various oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as
“fuming nitric acid”.
Chemical properties
A weak acid occurring only in the form of a light-blue solution.
Physical properties
Pale blue solution; stable only in solution; weak acid, Ka 4.5x10-4.
The Uses of NITROUS ACID
Nitrous acid is a nitrogen oxoacid. It is a conjugate acid of a nitrite. It (as sodium nitrite) is used as part of an intravenous mixture with sodium thiosulfate to treat cyanide poisoning. There is also research to investigate its applicability towards treatments for heart attacks, brain aneurysms, pulmonary hypertension in infants, and Pseudomonas aeruginosa infections.
The Uses of NITROUS ACID
Formation of diazotizing compounds by reaction with primary aromatic amines, source of nitric oxide.
Indications
For sequential use with sodium thiosulfate for the treatment of acute cyanide poisoning that is judged to be life-threatening .
Background
Nitrous acid (as sodium nitrite) is used as part of an intravenous mixture with sodium thiosulfate to treat cyanide poisoning. It is on the World Health Organization's List of Essential Medicines, a list of the most important medications needed in a basic health system. There is also research to investigate its applicability towards treatments for heart attacks, brain aneurysms, pulmonary hypertension in infants, and Pseudomonas aeruginosa infections.
Definition
A weak acid known only in solution, obtained by acidifying a solution of a nitrite. It readily decomposes on warming or shaking to nitrogen monoxide and nitric acid. The use of nitrous acid is very important in the dyestuffs industry in the diazo reaction: nitrous acid is liberated by acidifying a solution of a nitrite (usually sodium nitrite) in the presence of the compound to be diazotized. Nitrous acid and the nitrites are normally reducing agents but in certain circumstances they can behave as oxidizing agents, e.g. with sulfur dioxide and hydrogen sulfide.
Definition
nitrous acid: A weak acid, HNO2,known only in solution and in thegas phase.It is prepared by the actionof acids upon nitrites, preferablyusing a combination that removesthe salt as an insoluble precipitate(e.g.Ba(NO2)2 and H2SO4). The solutionsare unstable and decompose on heating to give nitric acid and nitrogenmonoxide.Nitrous acid can functionboth as an oxidizing agent(forms NO) with I– and Fe2+, or as areducing agent (forms NO3-) with,forexample, Cu2+; the latter is mostcommon.It is widely used (preparedin situ) for the preparation of diazoniumcompounds in organic chemistry.The full systematic name isdioxonitric(III) acid.
Preparation
Nitrous acid may be obtained in solution by adding a strong acid to nitrite; e.g., adding hydrochloric acid to sodium nitrite solution:
H+ + NO2 ˉ → HNO2.
Hazard
Rapidly forms nitric oxide and nitric acid in water; [Merck Index] A strong oxidizer; Causes burns; Highly toxic by ingestion and inhalation.
Pharmacokinetics
Sodium nitrite reverses cyanide toxicity and produces blood vessel dilation .
Safety Profile
Mutation data reported. Flammable by chemical reaction; a powerful oxidizer. Explodes on contact with phosphorus trichloride. Reacts violently with PH3 and Pcb. Reactions with l-amino- 5-nitrophenol, ammonium decahydroborate(2-), hydrazine (product is hydrogen azide) may give explosive products. Incompatible with anilines (e.g., 4- bromoahe , 2-chloroaniline, 3- chloroaniline, 2-nitroadine, 3-nitroaniline, 4-nitroaniline, aniline ), semicarbazone, silver nitrate. When heated to decomposition it emits hghly toxic fumes of NOx. See also NITRIC OXIDE.
Metabolism
Reduced by deoxyhemoglobin to form nitric oxide . Nitrite is also reduced to nitric oxide and further reduced to ammonia by gut bacteria . Nitrite can be oxidized to nitrate by oxyhemoglobin.
Properties of NITROUS ACID
| Density | 1.54±0.1 g/cm3(Predicted) |
| pka | pK (25°) 3.35 |
| form | stable only in solution |
| color | stable only in solution |
| PH | 3.28(1 mM solution);2.67(10 mM solution);2.13(100 mM solution) |
| EPA Substance Registry System | Nitrous acid (7782-77-6) |
Safety information for NITROUS ACID
Computed Descriptors for NITROUS ACID
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1