Nitrohydrochloric acid
- CAS NO.:8007-56-5
- Empirical Formula: ClHNO2
- Molecular Weight: 82.46644
- MDL number: MFCD01742234
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
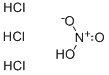
What is Nitrohydrochloric acid?
Chemical properties
Aqua regia, also known as nitrohydrochloric acid and chloroazoatic acid, is a fuming,volatile liquid that is made by mixing three parts concentrated hydrochloric acid with one part concentrated nitric acid.It is very corrosive with a suffocating odor and reacts with all metals. Aqua regia typically reacts by oxidizing the metal to a metallicion and reducing the nitric acid to nitric oxide.Its reaction with silver produces silver chloride. Aquaregia dissolves the common metallic oxides and hydroxides, the ignited oxides of tin, aluminum,chromium, and iron, and the higher oxides of lead, cobalt,nickel,and manganese. It is used for testing gold and platinum.
The Uses of Nitrohydrochloric acid
Metallurgy, testing metals, dissolving metals.
Definition
A mixture of concentrated nitric acid and three to four parts of hydrochloric acid. It dissolves all metals including gold, hence the name. The mixture contains chlorine and NOCl (nitrosyl chloride).
General Description
A yellow liquid with a pungent odor prepared by mixing nitric acid and hydrochloric acid, usually in a ratio of one part of nitric acid to three or four parts of hydrochloric acid. Fumes are irritating to the eyes and mucous membranes. Corrosive to metals and to tissue. Density 14.7 lb /gal.
Air & Water Reactions
Fumes in air. Soluble in water with release of heat.
Reactivity Profile
Nitrohydrochloric acid is a powerful oxidizing agent and a strong acid. Reacts exothermically with chemical bases (for example: amines and inorganic hydroxides) to form salts and water. Reacts with most metals, including gold and platinum, to dissolve them with generation of toxic and/or flammable gases. Can initiate polymerization in polymerizable organic compounds. Reacts with cyanide salts to generate toxic hydrogen cyanide gas. Generates flammable and/or toxic gases with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and weak or strong reducing agents. Additional exothermic gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and carbonates (CO2).
Hazard
A powerful oxidizer, toxic, corrosive liquid.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Agricultural Uses
Aqua regia is an extremely effective oxidizing solvent, capable of dissolving metals like gold and platinum. It is made of a mixture of one part concentrated nitric acid and three parts hydrochloric acid.
Properties of Nitrohydrochloric acid
| Stability: | Pressure will develop in sealed bottles. Incompatible with (and may react violently with) a wide variety of materials, including metals, oxidising agents, reducing agents, combustible materials. |
| CAS DataBase Reference | 8007-56-5 |
| EPA Substance Registry System | Aqua regia (8007-56-5) |
Safety information for Nitrohydrochloric acid
Computed Descriptors for Nitrohydrochloric acid
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
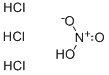

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1