NITROFEN
Synonym(s):2,4-Dichlorophenyl 4-nitrophenyl ether;TOK
- CAS NO.:1836-75-5
- Empirical Formula: C12H7Cl2NO3
- Molecular Weight: 284.09
- MDL number: MFCD00128026
- EINECS: 217-406-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
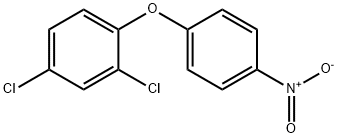
What is NITROFEN?
Chemical properties
Nitrofen is a crystalline solid.
The Uses of NITROFEN
Formerly as herbicide.
Definition
ChEBI: Nitrofen is an organic molecular entity. It has a role as an EC 1.3.3.4 (protoporphyrinogen oxidase) inhibitor and a herbicide.
General Description
Colorless crystals or black solid. Used as a pre- or post-emergence herbicide.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
NITROFEN is a nitrated and halogenated ether derivative.
Health Hazard
Nitrofen is moderately toxic by ingestionand inhalation of dusts. The lethal doses in cats from oral administration and inhalationof dusts are 300 mg/kg and 620 mg/m3/4h,respectively (NIOSH 1986). Bovine calvestreatedorally by 1.5 mL 25% nitrofen/kg produced toxic effects after 36–48 hours. Thesymptoms were increase in body temperature, depression, and progressive decrease inrespiration rate and pulse rate,similar to tribulin (Gupta and Singh 1985). An increase inthe activities of serum glutamic-oxaloacetictransaminase and glutamipyruvictransaminase was noted(Gupta and Singh 1984). Nitrofen has beenfound to cause cancer in animals. There issufficient evidence of its carcinogenicity inanimals (IARC). Oral administration in micecaused liver and lung cancers.
Fire Hazard
Flash point data for NITROFEN are not available; however, NITROFEN is probably combustible.
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Poison by ingestion. Moderately toxic by inhalation and possibly other routes. Experimental teratogenic and reproductive effects. A skin and severe eye irritant. Mutation data reported. A broad-spectrum herbicide. See also NITRO COMPOUNDS of AROMATIC HYDROCARBONS and ETHERS. When heated to decomposition it emits very toxic fumes of Cl and NOx.
Potential Exposure
Nitrofen is a contact herbicide used for pre-and post-emergency control of annual grasses and broadleaf weeds on a variety of food and ornamental crops. Occupational exposure to nitrofen, primarily through inhalation and dermal contact may occur among workers at production facilities. Field handlers of the herbicide are subject to inhalation exposure during application procedures.
Carcinogenicity
Nitrofen is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Shipping
UN3345 Phenoxyacetic acid derivative pesticide, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard Class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Waste Disposal
Small quantities may be landfilled but large quantities should be incinerated. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of NITROFEN
| Melting point: | 69-70°C |
| Boiling point: | 368 ºC |
| Density | 1.3 |
| refractive index | 1.6140 (estimate) |
| Flash point: | 205℃ |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | soluble in Methanol |
| form | Solid |
| color | White to Light yellow |
| Water Solubility | 1mg/L(22 ºC) |
| Merck | 14,6598 |
| BRN | 1887356 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| NIST Chemistry Reference | 2,4-Dichloro-4'-nitrodiphenyl ether(1836-75-5) |
| IARC | 2B (Vol. 30, Sup 7) 1987 |
| EPA Substance Registry System | Nitrofen (1836-75-5) |
Safety information for NITROFEN
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H350:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for NITROFEN
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 2,4-Dichloro-4'-nitrobiphenyl Ether CAS 1836-75-5View Details
2,4-Dichloro-4'-nitrobiphenyl Ether CAS 1836-75-5View Details
1836-75-5 -
 TOKUNAGA human cell line CASView Details
TOKUNAGA human cell line CASView Details -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4