Nitrocellulose
- CAS NO.:9004-70-0
- Empirical Formula: C24H36N8O38
- Molecular Weight: 1044.57344
- MDL number: MFCD00081525
- EINECS: 933-629-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:59
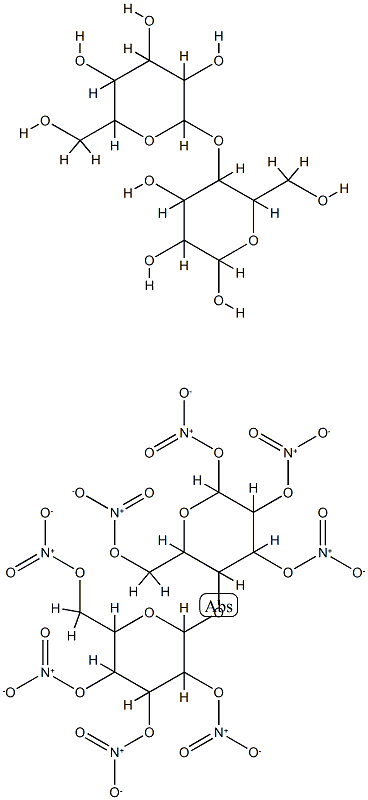
What is Nitrocellulose?
Description
Early explosives used nitric acid. They were so unstable to handle
that European scientists tried to find an explosive compound for
better safety. Around 1846, it was discovered that concentrated
nitric acid absorbed into cotton was not explosive until dried;
thus, guncotton was developed by chemical binding of nitrate
to cellulose. Preventing spontaneous explosions during the
manufacturing process required extensive washing and drying of
the cotton. In 1884, a French chemist made smokeless powders
from nitrocellulose. Their more stable and slower burning
properties enabled development of firearms and artillery
ammunitions. Eastman Kodak film products used nitrocellulose
as early as 1889. The film was used until 1933 for X-ray films and
for motion picture films until 1951.
Nitrocellulose can take on various physical forms, from
white fibers to thin sheets to thick liquid. Nitrocellulose can
also be a white, yellow, or transparent plastic. Its rigidity varies
from brittle to flexible. The unique properties enable nitrocellulose
to be used now in a wide variety of products. The variability
in physical properties comes from the content of
nitrogen and determines the use. The molecular weight of
nitrocellulose ranges from 459.28 to 594.28, and the molecular
formula is expressed as [C6H7O2(ONO2)3]n. The hydroxyl
group of glucose units react to form nitrocellulose chains and
membranes.
Nitrocellulose is thus a fibrous solid polymer consisting of
the cellulose ester of nitric acid. Its specific gravity is 1.66. Its
form can be a white pulpy, cotton-like, amorphous solid in the
dried state, or a colorless liquid to semisolid, depending on the
degree of nitration. It has low water solubility; however, it is
soluble in 25% of a mixture of 1 volume of alcohol and 3
volumes of ether, forming collodion. Nitrocellulose is also
soluble in organic solvents such as methanol, acetone, glacial
acetic acid, and amyl acetate.
Chemical properties
pale yellow syrupy liquid
Chemical properties
Nitrocellulose is a pulpy, cotton-like solid, or a colorless liquid solution.
The Uses of Nitrocellulose
Nitrocellulose is used as a propellant in artillery ammunition, in small-arms ammunition, in chemical explosives, and in smokeless powder. It is made by reacting cotton with nitric acid.
The Uses of Nitrocellulose
In manufacture of collodions; in lacquer coatings, inks, adhesives. Cellulose hexanitrate is used in explosives and propellants. Celloidin is used for embedding sections in microscopy; in electrotechnics, photography, galvanoplasty.
The Uses of Nitrocellulose
Products using nitrocellulose range from a strong, resistant plastic to an unstable class B (highly flammable, explosive when confined) explosive material. The major products include smokeless gun powder, waterproof fuses in pyrotechnics, inks, adhesives, varnishes, resins, lacquer coatings, embedding sections in microscopy, photography, and plastics. Nitrocellulose membranes are used to immobilize DNA, RNA, or protein to probe with a labeled sequence or antibody in experimental laboratory methods such as Western blotting. Other uses include skin protectants for cosmetics and microfilters. Nitrocellulose is also currently utilized in photography, lacquers, patent and natural leathers, artificial pearls, process engraving, and cements. Guncotton dissolved in a 25% acetone solvent can be used for wood finishing, providing deep luster.
Definition
cellulose nitrate: A highly flammablematerial made by treating cellulose(wood pulp) with concentratednitric acid. Despite the alternativename nitrocellulose, the compound isin fact an ester (containing CONO2groups), not a nitro compound(which would contain C–NO2). It isused in explosives (as guncotton) andcelluloid.
Definition
Collodion: a thin film of cellulosenitrate made by dissolving the cellulosenitrate in ethanol or ethoxyethane,coating the surface, andevaporating the solvent.
Preparation
Cellulose nitrate is prepared according to the following reaction:
C6H10O5+HNO3-->[-C6H7O2(OH)(ONO2)2-]n
The nitrogen content for plastics is usually about 11%, for lacquers and cement base it is 12%, and for explosives it is 13%. The standard plasticizer added is camphor.
Key properties of cellulose nitrate are good dimensional stability, low water absorption, and toughness. Its disadvantages are its flammability and lack of stability to heat and sunlight.
General Description
Pale yellow syrupy liquid with an ether-like odor. Floats on water. Produces an irritating vapor. Immiscible with water. Boiling point is around 94°F.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Nitrocellulose is a solution of pyroxylin in ether and alcohol with a flash point of approximately 0°F.
Hazard
Flammable, dangerous fire and explosion risk. Somewhat less flammable when wet.
Health Hazard
High concentration of ether fumes may cause narcosis, loss of consciousness and respiratory paralysis if inhaled. Contact with eyes causes irritation.
Fire Hazard
Nitrocellulose is a white fibrous solid or amorphous powder. It is wetted with water, alcohol, or other solvent for handling and storage. It may be made to various forms, gel, flake, granular, or powder. Dry material is a low explosive and often used in combination with another explosive, such as nitroglycerine, to obtain more brisance for the composition. Dry nitrocellulose does not detonate but deflagrates. When wetted with water or alcohol, its sensitivity is considerably reduced.
Nitrocellulose presents three types of hazards. As mentioned earlier, it is an explosive compound. It explodes upon burning or friction. It is a flammable solid having a flash point of 13°C (55 °F). It can therefore ignite at ambient temperatures, thus presenting a severe fire and explosion hazard. It burns at a very rapid rate. The combustion products consist of extremely toxic gases: notably, hydrogen cyanide, carbon monoxide, and oxides of nitrogen.
Fires involving nitrocellulose should be fought with extreme caution. Unmanned fixed turrets and hose nozzles should be used. Since nitrocellulose produces oxygen on decomposition, a large volume of water should be applied through spray nozzles to cool the material and wet the entire surface. Self-contained breathing apparatus must be worn by firefighters for protection against highly toxic gases.
.
Agricultural Uses
Cellulose nitrate is a highly flammable material made by treating cellulose (wood pulp) with a mixture of concentrated nitric acid and concentrated sulphuric acid. Despite the alternative name, nitrocellulose, cellulose nitrate is an ester of cellulose containing -ONO2 groups and is not a nitro compound. It is used in explosives and celluloid.
Agricultural Uses
When cellulose is acidulated with a mixture of concentrated nitric acid and sulphuric acid, nitrocellulose is formed, which is also called cellulose nitrate. The nitrocellulose is used in explosives and celluloid, since it is highly inflammable. Nitrocellulose is not a nitro compound, but an ester containing -CONO2groups.
Industrial uses
Cellulose nitrates are materials made by treatingcellulose with a mixture of nitric and sulfuricacids, washing free of acid, bleaching,stabilizing, and dehydrating. For sheets, rods, and tubes it is mixed with plasticizers and pigmentsand rolled or drawn to the shape desired.The lower nitrates are very inflammable, butthey do not explode like the high nitrates, andthey are the ones used for plastics, rayons, andlacquers, although their use for clothing fabricsis restricted by law. The names cellulose nitrateand pyroxylin are used for the compounds oflower nitration, and the term nitrocellulose isused for the explosives.The outstanding toughness properties ofcellulose nitrate lead to its continuing use insuch applications as optical frames, shoe eyelets,ping pong balls, and pen barrels.
Safety Profile
Very low oral toxicity. Flammable solid. Highly dangerous fire hazard in the dry state when exposed to heat, flame, or powerful oxidizers. When wet with 35% of denatured ethanol it is about as hazardous as ethanol alone or gasoline. Dry cellulose tetranitrate burns rapidly with intense heat and ignites easily. Moderately dangerous explosion hazard. To fight fire, use copious volumes of water; alcohol foam. CO2 is effective in extinguishing fires of nitrocellulose solvents. See also EXPLOSIVES, HIGH.
Potential Exposure
It is used in making explosives, rocket propellants and celluloid.
Environmental Fate
The physicochemical properties of nitrocellulose limit dispersion
throughout the environment. In addition to those noted
earlier, the melting point of the solid is relatively high (between
169 and 170℃), the flash point is 12.8 ℃, and nitrocellulose
is nonvolatile. Nitrocellulose is insoluble in water, and thus is
not detected in high concentrations in water, and tends to stay
on sediments. Direct decomposition of nitrocellulose is
unlikely, as the degradation process needs a severe chemical
reaction like alkaline hydrolysis to break its beta 1 → 4-
glucoside units bond linkages.
Because of its particulate character, specific gravity, and
low solubility, nitrocellulose particles tend to accumulate in
sediments in aqueous systems. The settled particles are very
stable and resistant to degradation for long periods of time.
They appear to remain unchanged in the environment. Their
biodecomposition by microorganisms is also not likely. No
studies are available on their long-range transport.
Storage
Nitrocellulose should be stored as a wetted substance and never allowed to go dry. Storage should be in a cool, well-entilated location isolated from all heat sources. Shipping should be in steel drums or barrels wet with 25-35% alcohol, water, or other solvent.
Shipping
UN2555, UN2556 and UN2557 requires a shipping label of “FLAMMABLE SOLID.” They fall in DOT Hazard Class 4.1. UN0342 and UN0343 requires a shipping label of “EXPLOSIVE.” They fall in DOT Hazard Class 1.3C.
Incompatibilities
Dust and powder form explosive mixture with air. Keep wet; do not allow to become dry. Dry material is a shock-sensitive explosive. Desensitize using water or alcohol. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Properties of Nitrocellulose
| Melting point: | 100 °C |
| Boiling point: | 83 °C(lit.) |
| Density | 1.23 g/mL at 25 °C(lit.) |
| refractive index | 1.6081 (estimate) |
| Flash point: | 53 °F |
| storage temp. | 0-6°C
|
| solubility | esters, ketones, ether-alcohol mixtures (collodion) and glycol ethers: soluble |
| form | Viscous Liquid |
| color | Clear colorless to light yellow |
| Specific Gravity | 0.765~0.775 |
| PH | pH(25℃):4.0~8.0 |
| Dielectric constant | 6.2(Ambient) |
| Stability: | Stable. Extremely flammable - presents a serious fire risk. Note the very low flash point and extremely wide explosive limits. Readily forms an explosive mixture with air. Incompatible with oxidizing agents, strong acids, strong bases, amines |
| EPA Substance Registry System | Nitrocellulose (9004-70-0) |
Safety information for Nitrocellulose
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H226:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. |
Computed Descriptors for Nitrocellulose
Nitrocellulose manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Nitrocellulose powder CAS 9004-70-0View Details
Nitrocellulose powder CAS 9004-70-0View Details
9004-70-0 -
 Collodion 4% CAS 9004-70-0View Details
Collodion 4% CAS 9004-70-0View Details
9004-70-0 -
 Collodion flexible CAS 9004-70-0View Details
Collodion flexible CAS 9004-70-0View Details
9004-70-0 -
 COLLODION FLEXIBLE CASView Details
COLLODION FLEXIBLE CASView Details -
 Collodion solution CAS 9004-70-0View Details
Collodion solution CAS 9004-70-0View Details
9004-70-0 -
 Cellulose Nitrate (Nitrocellulose), CAS Number: 9004-70-0View Details
Cellulose Nitrate (Nitrocellulose), CAS Number: 9004-70-0View Details
9004-70-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
