Nisin
Synonym(s):Nisaplin
- CAS NO.:1414-45-5
- Empirical Formula: C143H230N42O37S7
- Molecular Weight: 3354.07
- MDL number: MFCD16661185
- EINECS: 215-807-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
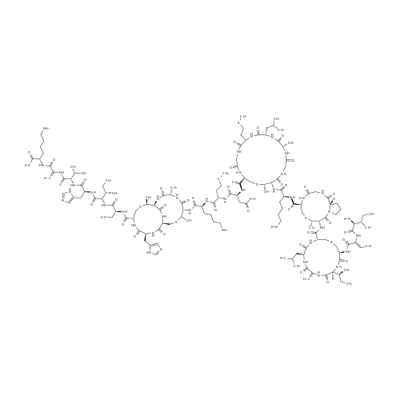
What is Nisin?
Chemical properties
Crystals from ethanol. Derived from Streptococcus lactis Lancefield Group N.
The Uses of Nisin
Nisin is a particularly effective bacteriosin against C. botulinum, is allowed in processed cheese food.
The Uses of Nisin
Nisin is an antimicrobial agent derived from pure culture fermenta- tions of certain strains of streptococcus lactis lancefield group n. nisin preparation contains nisin, a group of related peptides with antibiotic activity. it is used to inhibit the outgrowth of clostridium botulinum spores and toxin formation in pasteurized cheese spreads and pasteurized process cheese spreads; pasteurized cheese spread with fruits, vegetables, or meats; and pasteurized process cheese spread with fruits, vegetables, or meats.
The Uses of Nisin
An antibiotic with bactericidal action
Definition
ChEBI: A type-A lantibiotic containing 34 amino acid residues (including lanthionine (Lan), methyllanthionine (MeLan), didehydroalanine (Dha) and didehydroaminobutyric acid (Dhb)) and five thioether bridges. It is obtained by fermentation of the bacterium L ctococcus lactis and shows particular activity against Clostridium botulinum. It is used in the production of various processed foods to suppress Gram-positive spoilage and pathogenic bacteria and so extend shelf life.
What are the applications of Application
Nisin from Lactococcus lactis is Nisin from Lactococcus lactis binds to cell wall precursor lipid components (lipid A portion) of bacteria and disrupts cell wall production. Nisin alters the cell membrane which results in the leakage of cytoplasmic components and destruction of the proton motive force1.
Biological Activity
nisin is an antibacterial peptide.nisin is produced by the lactic acid bacterium l. lactis using uncommon amino acids, including lanthionine, and is a member of the class of antibiotics referred to as lantibiotics.
Biochem/physiol Actions
Nisin (Nisaplin) is produced by Lactococcus lactis. It is an effective lantibiotic peptide bactericidal agent. Nisin is a broad spectrum antimicrobial bacteriocin which affects Gram-positive bacteria, however, it has little or no effect on Gram-negative bacteria, yeasts or fungi.It was demonstrated that nisin has capability to inhibit the growth of almost all tested Gram-positive beer-spoilage bacteria. Of 122 Gram-positive bacteria strains (predominantly Lactobacilli and Pediococci), 93% were sensitive to 100 units of nisin. In contrast, out of the 32 Gram-negative strains tested, only the genus Flavobacter proved to be affected by nisin.Nisin exerts its antimicrobial activity by binding to lipid II which is a membrane-bound advanced intermediate involved in the biosynthesis of bacterial cell wall. By doing so, nisin sequesters lipid II from its functional location. In addition, the nisin-lipid II complex leads to formation of pores in the membrane causing cell death.
Mechanism of action
Nisin is highly effective against Gram-positive bacteria, with its MICs being at nanomolar concentrations. There are two different mechanisms by which nisin kills bacteria: pore formation in the membrane and inhibition of cell wall biosynthesis by binding to lipid II. After nisin reaches the bacterial plasma membrane, a pyrophosphate cage which involves the first two rings of nisin and the pyrophosphate moiety of lipid II is formed via hydrogen bonds. The pyrophosphate is responsible for the low levels of resistance of bacteria to nisin, since the pyrophosphate is essential and not prone to mutation and also facilitates the transmembrane orientation of nisin. Nevertheless, it is difficult for nisin to penetrate the outer membrane barrier of Gram-negative bacteria, and thus, it cannot reach its target, lipid II, in the inner membrane. This leads to the relatively low level of activity of nisin against Gram-negative bacteria. Conversely, nisin actually tends to bind to the usually anionic surface of the outer membrane and stabilizes it via electrostatic interactions. Notably, nisin can inhibit the growth of Gram-negative bacteria more efficiently, when chelating agents (EDTA, citrate monohydrate, or trisodium orthophosphate) are used to destabilize the outer membrane. Thus, the main bottleneck for nisin to be active against Gram-negative bacteria appears to be its ability to pass the outer membrane[2].
Side Effects
Although Nisin E234 generally regarded as a very safe and effective supplement, there can be some minor side effects. Possible side effects: pruritus, nausea, and flushing. Other side effects include: pruritus, skin rash, and vomiting.
in vitro
previous study showed that the sperm motility could be completely inhibited with nisin the minimum effective concentration of nisin required to immobilize sperm in vitro within 20 s was found to be 50 μg in rat, 200 μg in rabbit and 300-400 μg in monkey and human. such inhibitory effect on sperm motility was found to be dose- and time-dependent [1].
in vivo
intravaginal administration of nisin before mating during proestrus-estrous transition phase caused complete arrest of sperm motility and blockage of conception. subacute toxicity studies in rats showed that, repetitive intravaginal application of nisin at the dose of 200 microg for 14 days caused no abnormalities either in the length of estrous cycle or in the morphology of vaginal epithelial cells. moreover, no histopathological abnormalities in vaginal tissue or any change in blood and serum biochemical profiles were seen. in addition, no adverse effects were found on subsequent reproductive performance, development of pups and neonate survival [1].
Purification Methods
This polypeptide from S. lactis is purified by crystallisation from 80% (v/v) EtOH and by countercurrent distribution. The synthetic polypeptide antibiotic can also be purified by preparative HPLC and assayed by HPLC on a Nucleosil 3007C18 (6 x 250mm) column using a MeCN—0.01M HCl gradient (30-50%), at 2%/minute, and flow rate of 1.5mL/minute to give a retention time of 8.1 minutes; or MeCN—0.3M guanidine-HCl gradient (30-50%), at 2%/minute, and flow rate of 1.5ml/minute to give a retention time of 10.9minutes. FAB-MS gave the pseudomolecular ion m/z at 3352.7 (M + H)+. It is soluble in dilute acid and is stable even on boiling. [Berridge et al. Biochem J 52 529 1952, synthesis by Fukase et al. Tetrahedron Lett 29 795 1988.]
References
[1] aranha c, gupta s, reddy kv. contraceptive efficacy of antimicrobial peptide nisin: in vitro and in vivo studies. contraception. 2004 apr;69(4):333-8.
[2] Li Q, et al. Increasing the Antimicrobial Activity of Nisin-Based Lantibiotics against Gram-Negative Pathogens. Applied and Environmental Microbiology, 2018; 84: e00052-18.
Properties of Nisin
| Boiling point: | 2967℃ |
| Density | 1.02 g/mL |
| Flash point: | >110°(230°F) |
| storage temp. | -20°C |
| solubility | insoluble in H2O; insoluble in EtOH; ≥5.63 mg/mL in DMSO with gentle warming and ultrasonic |
| form | solution |
| color | Off-white to light brown |
| Water Solubility | Soluble in water |
| Merck | 13,6592 |
| CAS DataBase Reference | 1414-45-5 |
Safety information for Nisin
Computed Descriptors for Nisin
| InChIKey | NVNLLIYOARQCIX-QSXKFZIVNA-N |
Nisin manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 1414-45-5 Nisin 98%View Details
1414-45-5 Nisin 98%View Details
1414-45-5 -
 Nisin from Lactococcus lactis CAS 1414-45-5View Details
Nisin from Lactococcus lactis CAS 1414-45-5View Details
1414-45-5 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1