Nimorazole
Synonym(s):4-(2-(5-Nitro-1H-imidazol-1-yl)ethyl)morpholine;
- CAS NO.:6506-37-2
- Empirical Formula: C9H14N4O3
- Molecular Weight: 226.23
- MDL number: MFCD00866796
- EINECS: 229-394-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
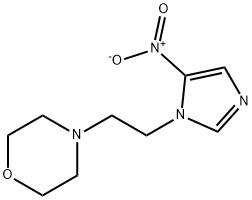
What is Nimorazole?
Description
Nimorazole is 5-nitroimidazole with antimicrobial and radiosensitizing activities. It is active against T. vaginalis in a Coulter counter antimicrobial assay (EC50 = 2.55 fmol/cell) and G. intestinalis (IC50 = 0.393 μM). Nimorazole reduces the number of vaginal trichomonads in a mouse model of T. vaginalis intravaginal infection (ED50 = 5.62 mg/kg). Under hypoxic conditions, nimorazole enhances radiation-induced SCC-7 tumor cell death in vitro and in vivo.
Originator
Naxogin,Carlo Erba,UK,1970
The Uses of Nimorazole
Nimorazole is an antimicrobial with activity against anaerobic bacteria and protozoa.
Definition
ChEBI: Nimorazole is a member of imidazoles and a C-nitro compound.
Manufacturing Process
6 g 4(5)-nitroimidazole sodium salt and 9 g β-chloroethylmorpholine are
allowed to react in 200 ml dry toluene. The mixture is refluxed for 50 hours,
then cooled and filtered from the solid residue. The solvent is evaporated
under reduced pressure. The half-solid product thus obtained solidifies by
addition of petroleum ether and ethyl ether.
Crystallization from water results in N-β-ethylmorpholino-(5)-nitroimidazole
(melting point 110°C to 111°C); from mother liquors N-β-ethylmorpholino-
(4)-nitroimidazole (melting point 104°C to 106°C) is obtained.
The following procedure is given in US Patent 3,458,528: 78 grams (0.675
mol) of 5-nitroimidazole is dissolved in 1,500 ml of acetic acid upon the
addition of 72 ml (0.57 mol) of boron trifluoride etherate. 175 ml (3.5 mols)
of ethylene oxide in 175 ml of hexane, in a dropping funnel topped with a cold finger, is added slowly over 1 hour to the above solution maintained at 32° to
35°C with a water cooling bath. The mixture is concentrated under high
vacuum to 100 to 150 ml volume. The residue is diluted with 500 ml of water,
neutralized to pH 7 with aqueous sodium hydroxide, and extracted with 1.5
liters of ethyl acetate. The extract is dried and evaporated to yield 1-(2'-
hydroxyethyl)-5-nitroimidazole.
20 grams (0.127 mols) of 1-(2'-hydroxyethyl)-5-nitroimidazole in 50 ml of dry
pyridine is reacted with 75 grams of p-toluenesulfonyl chloride at 15°C for 4
hours. The reaction mixture is poured into ice and water and the crystalline
precipitate is separated by filtration, washed with water and air dried to yield
1-(2'-p-toluenesulfonyloxyethyl)-5-nitroimidazole; MP 126° to 127°C.
16 grams, (0.057 mol) of 1-(2'-p-toluenesulfonyloxyethyl)-5-nitroimidazole
and 9.3 ml of morpholine are heated at 95°C for 4 hours. The reaction
mixture is taken up in water and extracted with ether. Evaporation of the
ether yields 1-(2'-N-morpholinylethyl)-5-nitroimidazole; MP 109° to 110°C.
Therapeutic Function
Trichomonacidal
Pharmaceutical Applications
An orally administered 5-nitroimidazole. It is slightly soluble in water at room temperature, soluble in alcohols, acetone and chloroform. The spectrum includes T. vaginalis, G. lamblia, E. histolytica, anaerobic bacteria and G. vaginalis. Activity against B. fragilis and Fusobacterium spp. is similar to or slightly less than that of metronidazole (mean MIC 0.25–1 mg/L).
A peak blood concentration of about 32 mg/L occurs within 2 h of a 500 mg oral dose. High concentrations are achieved in saliva and vaginal secretions. Excretion is principally via the urine where the drug appears as metabolites which display antimicrobial and antiprotozoal activity less than that of the parent drug.
It is generally well tolerated even at the high doses required in conjunction with radiotherapy for the treatment of head and neck tumors. Adverse effects are the same as those of metronidazole. Disulfiram-like reactions appear to be rare.
Clinical uses are similar to those of metronidazole. It is also used as a hypoxic radiosensitizer in the radiotherapy of head and neck tumors.
Properties of Nimorazole
| Melting point: | 110-111° |
| Boiling point: | 367.84°C (rough estimate) |
| Density | 1.2673 (rough estimate) |
| refractive index | 1.6300 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 6.65±0.10(Predicted) |
| form | Solid |
| color | Off-White to Yellow |
| CAS DataBase Reference | 6506-37-2 |
| NIST Chemistry Reference | Nimorazole(6506-37-2) |
Safety information for Nimorazole
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H340:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Nimorazole
Abamectin manufacturer
Ralington Pharma
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 6506-37-2 Nimorazole 98%View Details
6506-37-2 Nimorazole 98%View Details
6506-37-2 -
 Nimorazole 99% (HPLC) CAS 6506-37-2View Details
Nimorazole 99% (HPLC) CAS 6506-37-2View Details
6506-37-2 -
 Nimorazole CAS 6506-37-2View Details
Nimorazole CAS 6506-37-2View Details
6506-37-2 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4