Nicotine
Synonym(s):(−)-1-Methyl-2-(3-pyridyl)pyrrolidine;(-)-Nicotine;(S)-3-(1-Methyl-2-pyrrolidinyl)pyridine;3-(1-Methyl-2-pyrrolidinyl)pyridine, (S)-(-)-Nicotine
- CAS NO.:54-11-5
- Empirical Formula: C10H14N2
- Molecular Weight: 162.23
- MDL number: MFCD00006369
- EINECS: 200-193-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
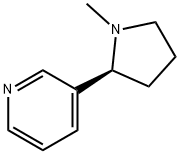
What is Nicotine?
Absorption
Absorption of nicotine through the buccal mucosa is relatively slow and the high and rapid rise followed by the decline in nicotine arterial plasma concentrations seen with cigarette smoking are not achieved with the inhaler. About 10% of absorbed nicotine is excreted unchanged in urine.
Toxicity
Symptoms of overdose include nausea, abdominal pain, vomiting, diarrhea, diaphoresis, flushing, dizziness, disturbed hearing and vision, confusion, weakness, palpitations, altered respiration and hypotension. LD50= 24 mg/kg (orally in mice).
Description
Nicotine is an alkaloid obtained from the dried leaves of Nicotiana tabacum and Nicotiana rustica. Nicotine stimulates acetylcholine receptors of the postsynaptic membrane at nerve synapses resulting in depolarization of the membrane. Toxic doses cause stimulation that is rapidly followed by blockade of nerve transmission.
Description
L-(–)-Nicotine is a dinitrogen alkaloid that is present in concentrations as high as 3% in the dried leaves of the tobacco plant (Nicotiana tabacum). It exists in even higher concentrations (up to 14%) in the lesser known “Aztec tobacco” (N. rustica).
Nicotine is an unusual alkaloid in that it has two nitrogen-containing heterocycles, pyridine and pyrrolidine. It is, of course, the tobacco component that makes smoking highly addictive, leading to the consequence that long-term smoking causes cancer. The now-famous 1964 Report of the Advisory Committee to the Surgeon General of the United States brought widespread attention to the dangers of smoking.
In the modern era, much nicotine is delivered via e-cigarettes. Smokers have found that the nicotine in e-cigarettes is not as harsh to inhale as the one contained in tobacco.
A recent report by David H. Peyton and co-workers at Portland State University (Oregon) suggests why this is so: Nicotine in e-cigarettes is primarily in its protonated form rather than the free base that exists in tobacco. The authors infer that protonated nicotine is easier to inhale. Thus, if anything, e-cigarettes are likely to increase nicotine addiction
If you think nicotine is bad when you inhale it, take a look at its acute effects shown in the hazard information box.
Description
(–)-Nicotine is an alkaloid that has been found in tobacco. It is an agonist at neuronal nicotinic acetylcholine receptors (nAChRs) and binds to α3β4 and α4β2 subunit-containing nAChRs with Ki values of 481 and 11.1 nM, respectively. Chronic exposure to (–)-nicotine results in increased expression of certain nAChRs, particularly α4β2 subunit-containing nAChRs. (–)-Nicotine has addictive properties. Formulations containing (–)-nicotine have been used as smoking cessation aids for the relief of nicotine withdrawal symptoms.
Chemical properties
Yellow Liquid
Chemical properties
Nicotine is a pale yellow to dark brown, oily liquid. Slight, fishy or pyridine-like odor when warm. It is also available as a powder.
Originator
Nicotinell TTS,Novartis
The Uses of Nicotine
(S)-(-)-Nicotine is a prototype nicotinic acetylcholine receptor agonist. (S)-(-)-Nicotine is the naturally occurring isomer. Nicotine can be absorbed through the alimentary canal, respiratory tract and intact skin. Nicotine is used in the treatment of smoking withdrawal syndrome. Nicotine has been used as an anthelmintic.
The Uses of Nicotine
Naturally-occuring isomer
The Uses of Nicotine
Prototype nicotinic acetylcholine receptor agonist; naturally occurring isomer.
The Uses of Nicotine
Nicotine is one of the principal constituentsof tobacco. It occurs in the dried leavesof Nicotiana tabacum and Nicotiana rusticato the extent of 2–8%. Exposure risk tothis alkaloid arises from smoking, chewing,or inhaling tobacco. Nicotine and its saltsare used as insecticides and fumigants, intanning, and in medicine.
The Uses of Nicotine
Nicotine is the second most widely used recreational drug after caffeine. At low doses, nicotineacts as a stimulant to the central nervous system by activating acetylcholine receptors, specifically called nicotinic acetylcholine receptors, in the postsynaptic neurons during nerve transmission.At higher doses nicotine acts as a depressant.Nicotine in tobacco has always been used for medicinal purposes. Nicotine solutions made from soaking tobacco leaves in water have been used as pesticides for several hundred years. In modern times, numerous pharmaceutical companies have explored nicotine's use for treating diseases. Nicotine's most prevalent medicinal use is for smoking cessation in the form of alternate delivery systems such as gums and dermal patches. Nicotine is used medically for numerous conditions and its use is being explored in additional areas including pain relievers, attention deficit disorder medications and medications associated with Alzheimer's disease, Parkinson disease, colitis, herpes, and tuberculosis.
Indications
For the relief of nicotine withdrawal symptoms and as an aid to smoking cessation.
Background
Nicotine is highly toxic alkaloid. It is the prototypical agonist at nicotinic cholinergic receptors where it dramatically stimulates neurons and ultimately blocks synaptic transmission. Nicotine is also important medically because of its presence in tobacco smoke.
Definition
ChEBI: An optically active form of nicotine having S-configuration.
Definition
A colourless poisonousalkaloid present in tobacco. It isused as an insecticide.
Production Methods
The nicotine molecule consists of a pyrrolidine ring attached to a pyridine ring by a bondbetween carbon atoms in the two-ring systems. Nicotine was isolated in impure form fromtobacco in 1809 by Louis Nicholas-Vauquelin (1763–1829). Vauquelin called the substancenicotianine. In 1826, Wilhelm Posselt (1806–1877) and Karl Ludwig Reimann (1804–1872),medical students at Heidelberg University, isolated pure nicotine and published dissertationson its pharmacology in 1828. Louis Henri Melsens (1814–1886) determined nicotine’sempirical formula. Amé Pictet (1857–1937) and P. Crépieux reported the synthesis of nicotine in 1903.
Manufacturing Process
The water extract from Nicotiana tabacum was prepared by distillation of
nicotine contained liquor from tobacco leaves, as described in D.R. Patent No.
319,846; September 12, 1913.
5 kg this water extract or the same quantity of tobacco powder in water was
mixed with 1.5 kg of grinded calcium hydroxide and 1.5 kg calcium sulfate.
The mixture stood for 24 hours. The obtained mixture looked like a dry
powder. It was extracted with ether. The ether was distilled and the residue
contented 98% of clear nicotine - liquid with odor of pyridine; BP: 246C/735
mm; d4
20 =1.0097; [α]d
20=- 166.5.
brand name
Habitrol (Novartis); Nicoderm (Sanofi Aventis); Nicotrol (Pharmacia & Upjohn); Prostep (Aveva).
Therapeutic Function
Ganglion depressant, Smoking deterrent
General Description
Liquids. Toxic by inhalation, ingestion, and skin absorption. More dense than water. Flash points usually below 140°F. Contact may irritate skin, eyes, and mucous membranes. If available, obtain the technical name of the material from the shipping papers and contact CHEMTREC, (800-424-9300) for specific response information.
Air & Water Reactions
Flammable. Slightly soluble in water.
Reactivity Profile
An alkaloid produced from tobacco. Colorless, oily liquid, combustible, highly toxic. When heated to decomposition L-Nicotine emits very toxic fumes of carbon monoxide and oxides of nitrogen [Lewis, 3rd ed., 1993, p. 919].
Hazard
Toxic by ingestion, inhalation, and skin absorption. Gastrointestinal damage, central nervous system impairment, and cardiac impairment
Health Hazard
L-Nicotine is classified as super toxic. Probable oral lethal dose in humans is less than 5 mg/kg or a taste (less than 7 drops) for a 70 kg (150 lbs.) person. It may be assumed that ingestion of 40-60 mg of nicotine is lethal to humans. There is fundamental difference between acute toxicity from use of nicotine as insecticide or from ingestion, and chronic toxicity that may be caused by prolonged exposure to small doses as occurs in smoking. Maternal smoking during pregnancy is associated with increased risk of spontaneous abortion, low birth weight and still-birth. Nicotine was found as a co-carcinogen in animals.
Health Hazard
Nicotine is a highly toxic compound. It stimulatesneuromuscular junctions and nicotinicreceptors, causing depression and paralysisof autonomic ganglia. Exposure routesare ingestion, absorption through skin, orinhalation (smoking or inhaling tobacco).The acute toxic symptoms in humansinclude nausea, vomiting, salivation, muscularweakness, twitching, and convulsions.The symptoms of confusion, hallucinations,and distorted perceptions have also beennoted in humans. Death may occur from respiratoryfailure. The lethal dose is approximately40 mg/kg. Chronic poisoning fromoccupational exposure may exhibit symptomsof vomiting and diarrhea. Fatal casesdue to occupational poisoning are unusual.
Nicotine administered in animals producedtoxic symptoms that include somnolence,change in motor activity, ataxia, dyspnea,tremor, and convulsions.
LD50 values, intraperitoneal (rats): 14.5 mg/kgLD50 values, oral (rats): 50 mg/kg
Kramer and coworkers (1989) investigatedthe effect of nicotine on the accumulation ofdopamine in synaptic vesicles prepared frommouse cerebral cortex or bovine striatum. Itwas found to be a weak inhibitor of dopamineaccumulation.
The role of nicotine in tobacco carcinogenesisis not yet fully understood. Nicotine is a precursor of N0-nitrosonornicotine , which is asuspected lung carcinogen (Hoffmann et al.1985). Nicotine-derived N-nitrosoaminescontribute significantly to carcinogenesiscaused by tobacco. However, there is noevidence in animals or humans of cancerscaused by nicotine itself. Berger and coworkers(1987) have found a beneficial effect ofperinatal nicotine administration in decreasingthe tumors of the neuorogenic systeminduced by N-methylnitrosourea in Sprague-Dawley rats. Although nicotineis noncarcinogenic, it acts as a cofactorin carcinogenesis induced by 7,12-dimethylbenz[a]anthracene in male Syrian goldenhamsters (Chen and Squier 1990).
Nicotine produced teratogenic effects intest animals, causing postimplantation mortality,fetal death, and developmental abnormalities.Nicotine and its primary metabolitecotinine exhibited teratogenic potential withXenopus frog embryo teratogenesis assay(Dowson et al. 1988).
Nicotine tested negative in the Neurosporacrassa –aneuploidy and histidinereversion–Ames tests for mutagenicity.
Fire Hazard
There is a moderate explosion hazard when exposed to heat or flame. When heated to decomposition, L-Nicotine emits nitrogen oxides, carbon monoxide and other highly toxic fumes. Avoid oxidizing materials. Stable under normal conditions. Avoid heat or flames.
Agricultural Uses
Insecticide: Nicotine is used in some drugs and insecticides. Classified for restricted use as an insecticide, limited to use by or under the direct supervision of a certified applicator. Not listed for use in EU countries. Registered for use in the U.S. and Canada. A U.S. EPA restricted Use Pesticide (RUP).
Trade name
BLACK LEAF®; CAMPBELL'S NICOSOAP ®; DESTRUXOL ORCHARD SPRAY®; EMONIB ®; FLUX MAAG®; FUMETO-TENDUST®; BAC®; MACH-NIC®; NIAGARA P. A. DUST®; NICODUST®; NICOFUME®; NICOCIDE®; ORTHO N-4 DUST®; XL ALL INSECTICIDE®
Pharmacokinetics
Nicotine, the primary alkaloid in tobacco products binds stereo-selectively to nicotinic-cholinergic receptors on autonomic ganglia, the adrenal medulla, neuromuscular junctions and in the brain. Nicotine exerts two effects, a stimulant effect exerted at the locus ceruleus and a reward effect in the limbic system. Itranvenous administration of nicotine causes release of acetylcholine, norepinephrine, dopamine, serotonine, vasopressin, beta-endorphin and ACTH. Nicotine is a highly addictive substance. Nicotine also induces peripheral vasoconstriction, tachycardia and elevated blood pressure. Nicotine inhalers and patches are used to treat smoking withdrawl syndrome. Nicotine is classified as a stimulant of autonomic ganglia.
Potential Exposure
An alkaloid produced from tobacco. Nicotine is used in some drugs; and in tanning. At one time, nicotine was used in the United States as an insecticide and fumigant; however, it is no longer produced or used in the United States for this purpose.
Carcinogenicity
Nicotine has low carcinogenic potential. One study found that diets containing 60 ppm nicotine and administered to rats for 300 days reduced the growth rate. The authors concluded that reduced body weight gains were only partially attributable to reduced food intake. No pathology on the rats and no evidence of carcinogenicity were reported. Rats were injected subcutaneously (5 days/week) for 26 weeks followed by an approximate 2-month observation period. Similarly, dogs were injected subcutaneously (5 days/week) for the same period. No tumors were observed in the test animals, although hyaline thickening and fibrosis of the vasculature of the kidney, lung, brain, and heart were evident.
Metabolic pathway
Nicotine has been used as an insecticide for at least 200 years but this naturally occurring compound lacks persistence and can be hazardous in use (Corbett et al., 1984). It has been replaced with more effective synthetic insecticides such as those in the neonicotinoid class. Most of the mformation on metabolism derives from research into the fate of nicotine after tobacco smoking as well as from the use of nicotine in agriculture and horticulture or through the biosynthesis of the alkaloid by plants and vegetables used as normal foodstuffs. Up to eight metabolites have been isolated and identified in man with six primary pathways. The main pathway is N-carbon oxidation of the pyrrolidine ring to form cotinine, others being N-oxidation of the pyrrolidine ring to form nicotine N-oxide, N-methylation of the pyridine ring to form an N-methylnicotinium ion and N-demethylation of the pyrrolidine ring to form nornicotine. Two other pathways are formation of a nicotine enamhe and of a nicotine glucuronide (Gabrielsson and Gumbleton, 1993). There is little information on the fate of nicotine in soil.
Metabolism
Primarily hepatic, cotinine is the primary metabolite.
Metabolism
Nicotine is well absorbed from the mucous membranes in the oral cavity, gastrointestinal tract, and respiratory system. If tobacco smoke is held in the mouth for 2 seconds, 66 to 77% of the nicotine in the smoke will be absorbed across the oral mucosa. If tobacco smoke is inhaled, approximately 90 to 98% of the nicotine will be absorbed. Nicotine is distributed throughout the body, readily crossing the blood-brain and placental barriers. The liver, kidney, and lung metabolize approximately 80 to 90% of the alkaloid. The kidney rapidly eliminates nicotine and its metabolites.
Shipping
UN1654 Nicotine, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
(-)-Nicotine is a very pale yellow hygroscopic oil with a characteristic odour (tobacco extract) which turns brown in air on exposure to light. It is purifed by fractional distillation under reduced pressure in an inert atmosphere. A freshly distilled sample should be stored in dark sealed containers under N2. It is a strong base; a 0.05 M aqueous solution has a pH of 10.2. It is very soluble in organic solvents. It is soluble in H2O and readily forms salts. [UV: Parvis J Chem Soc 97 1035 1910, Dobbie & Fox J Chem Soc 103 1194 1913.] The hydrochlorides (mono-and di-) form deliquescent crystals soluble in H2O and EtOH but insoluble in Et2O. It has also been purified via the ZnCl2 double salt. [Ratz Monatsh Chem 26 1241 1905, Biosynthesis: Nakan & Hitchinson J Org Chem 43 3922 1978.] The picrate has m 218o (from EtOH). [Beilstein 23/6 V 64.] POISONOUS.
Degradation
Nicotine is volatile and decomposes relatively quickly under the influence of light and air. The pyrrolidine nitrogen (pka 8.2) is more basic than the pyridine moiety (pka 3.1) (PM). Nicotine has a chiral centre, and exists naturally as the more potent (S) enantiomer.
Incompatibilities
Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Incompatible with strong acids. Attacks some forms of plastics, rubber, and coatings.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
References
Pictet, Rotschy., Ber., 33, 2533 (1900)
Tschitschibabin, Buchholz.,J. Russ. Phys. Chern. Soc., 50, 548 (1920)
Spath, Biniecki., Ber., 72, 1809 (1939)
Shmuk, Borozdina., CampI. rend. Acad. Sci., USSR, 12,1582 (1939)
Shmuk, Borozdina., J. Appl. Chern. Russ., 14,864 (1941)
Smith, Smith., J. Agric. Res., 65, 347 (1942)
Pal, Narasinham.,!. Ind. Chern. Soc., 20, 181 (1943)
Marion., Can. J. Res., 23B, 165 (1945)
Bottomley, Nottle, White., Austral. J. Sci., 8, 18 (1945)
Biosynthesis:
Leete.,!. Amer. Chern. Soc., 89,7081 (1967)
14C-NMR spectrum:
Ganz, Kelsey, Geiling., Bot. Gaz., 113, 195 (1951)
13C-NMR spectrum:
Crain, Wilderman, Roberts., J. Amer. Chern. Soc., 93,990 (1971)
Pharmacology :
Rolleston., Lancet., 210,961 (1926)
Laessing., Med. Welt., 12, 1485 (1938)
Coon, Rothman., Proc. Soc. Exp. Bioi. Med., 42, 231 (1939)
Straub, Amann., Klin. Woch., 19,169 (1940)
Coon, Rothman., J. Pharm. Exp. Ther. Froc., 72, 9 (1941)
Perlman, Dannesborg, Sokoloff., J. Amer. Med. Assoc., 120, 1003 (1942)
Roth, McDonald, Sheard., ibid, 125,761 (1944)
Burn, Truelove, Burn., Brit. Med. J., i, 403 (1945)
Properties of Nicotine
| Melting point: | -80 °C |
| Boiling point: | 243-248 °C |
| alpha | -166 º (c=neat) |
| Density | 1.010 g/mL at 20 °C(lit.) |
| vapor pressure | 0.06 hPa (20 °C) |
| refractive index | n |
| Flash point: | 215 °F |
| storage temp. | 2-8°C |
| solubility | ethanol: 50 mg/mL |
| form | liquid |
| appearance | colorless to yellow-brown oily liquid |
| pka | 8.02(at 25℃) |
| color | yellow |
| PH | 10.2 (8.1g/l, H2O, 20°C) |
| explosive limit | 0.7-4%(V) |
| optical activity | [α]20/D 169°(lit.) |
| Odor Threshold | 0.011ppm |
| Water Solubility | MISCIBLE |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,6524 |
| BRN | 82109 |
| Exposure limits | TLV-TWA air 0.5 mg/m3 (ACGIH, MSHA,
and OSHA). |
| CAS DataBase Reference | 54-11-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 3-(2-(N-methylpyrrolidinyl))pyridine(54-11-5) |
| EPA Substance Registry System | Nicotine (54-11-5) |
Safety information for Nicotine
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Nicotine
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 L-Nicotine 98% (GC) CAS 54-11-5View Details
L-Nicotine 98% (GC) CAS 54-11-5View Details
54-11-5 -
 (-)-Nicotine CAS 54-11-5View Details
(-)-Nicotine CAS 54-11-5View Details
54-11-5 -
 (−)-Nicotine CAS 54-11-5View Details
(−)-Nicotine CAS 54-11-5View Details
54-11-5 -
 Nicotex 2mg4mg SugarFree Mint Plus Flavour Nicotine Gum 29 Count NicoretteView Details
Nicotex 2mg4mg SugarFree Mint Plus Flavour Nicotine Gum 29 Count NicoretteView Details
54-11-5 -
 50L 95% Nicotine Alkaloid USP LiquidView Details
50L 95% Nicotine Alkaloid USP LiquidView Details
54-11-5 -
 Nicotine Alkaloid 95%, 1L-60LView Details
Nicotine Alkaloid 95%, 1L-60LView Details
54-11-5 -
 Pure Nicotine LiquidView Details
Pure Nicotine LiquidView Details
54-11-5 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
