Nickel(II) nitrate hexahydrate
Synonym(s):Nickel(II) nitrate hexahydrate
- CAS NO.:13478-00-7
- Empirical Formula: H12N2NiO12
- Molecular Weight: 290.79
- MDL number: MFCD00149805
- EINECS: 603-868-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
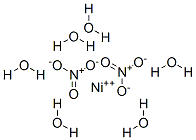
What is Nickel(II) nitrate hexahydrate?
Description
Nickel (II) nitrate hexahydrate appears as green crystals and substantially soluble in water. It is stable, a strong oxidiser, and incompatible with reducing agents. It has wide industrial applications in nickel plating as catalysts and is indirectly associated with other industries of nickel alloy manufacturers, nickel miners, smelters and refiners, nickel platers, and exposure to alloys, for example, in coinage.
Chemical properties
Green crystal
The Uses of Nickel(II) nitrate hexahydrate
Nickel plating, manufacturing of ceramic colorsNickel(II) nitrate hexahydrate is used as a precursor in the manufacturing of other nickel salts and supported nickel catalysts. It finds use as a catalyst in organic synthesis. Further it is used in electroplating and as a mordent in textile dyeing. It is a precursor for preparing nickel sulfide-reduced graphene oxide (RGO) composite powders with high initial capacities and good cycling performance. It can be employed in the preparation of nickel and nickel oxide nanocrystals impregnated O-MWCNTs (oxygen-functionalized multi-walled carbon nanotubes) with potential applications in semiconductor industries. It finds use in improving cycling stability ad rate capability of Li2MnO3.
The Uses of Nickel(II) nitrate hexahydrate
Nickel(II) nitrate hexahydrate may be used as a precursor for the synthesis of [Ni1/3Co1/3Mn1/3]O2 powders, Ni(II) oxide aerogels and crystalline nickel complexes, [Ni(C6H7N3O)2(NO3)2] and [Ni(C6H7N3O)3](NO3)2·H2O.
Preparation
Emerald green crystals of the hexahydrate Ni(N03)2?6H20 crystallize
from aqueous solutions at room temperature. These are isomorphous with the corresponding
cobalt(II) salt containing the regular octahedral [Ni(H20)6]2+ ions with Ni-O distances = 2.03 A. The tetrahydrate crystallize above 54° and the dihydrate above 85.4°.
The pale green anhydrous nitrate can be obtained by dehydration of the hydrate with
dinitrogen pentoxide in fuming nitric acid or by the liquid phase reaction of dinitrogen
tetroxide with nickel carbonyl [the gas phase reaction gives the nitrite Ni(N02)2]
Ni(CO)4+2N2O4 -* Ni(N03)2+2NO+4CO
The first product of this latter rather violent reaction is the adduct Ni(NO3)2N2O4 from
which the nitrogen tetroxide is removed by heating in vacuo. It can also be prepared by the
reaction of dinitrogen tetroxide-ethyl acetate mixtures with nickel(II) chloride. The infrared
spectrum shows it to be a typical covalent nitrate. It is involatile and decomposes upon
heating in nitrogen to give the nitrite Ni(NO2)2 above 260°.
General Description
Nickel(II) nitrate hexahydrate (Ni(NO3)2.6H2O) is a hydrated nickel(II) salt. Its thermal degradation has been investigated by thermogravimetric methods under quasi-isothermal conditions.
Safety Profile
Confirmed carcinogen. Moderately toxic by ingestion. When heated to decomposition it emits toxic fumes of NOx. See also NICKEL(II) NITRATE.
Purification Methods
Crystallise it from water (3.3g/mL) by partial evaporation in a desiccator. Store it in a desiccator as it is deliquescent.
Properties of Nickel(II) nitrate hexahydrate
| Melting point: | 56 °C(lit.) |
| Boiling point: | 137 °C |
| Density | 2.05 g/mL at 25 °C(lit.) |
| Flash point: | 137°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 940g/l |
| form | flakes |
| Specific Gravity | 2.05 |
| color | Green to blue |
| PH | 5 (50g/l, H2O, 20℃) |
| Water Solubility | 238.5 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,6513 |
| Exposure limits | ACGIH: TWA 0.1 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 |
| Stability: | Stable. Strong oxidizer. Incompatible with reducing agents. |
| CAS DataBase Reference | 13478-00-7(CAS DataBase Reference) |
Safety information for Nickel(II) nitrate hexahydrate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H315:Skin corrosion/irritation H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H334:Sensitisation, respiratory H341:Germ cell mutagenicity H350:Carcinogenicity H360:Reproductive toxicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Nickel(II) nitrate hexahydrate
| InChIKey | AOPCKOPZYFFEDA-UHFFFAOYSA-N |
Nickel(II) nitrate hexahydrate manufacturer
JSK Chemicals
ARRAKIS INDUSTRIES LLP
New Products
Ethyl 3,3- diethoxypropionate 7-Bromo-2-chloroquinoxaline N-Benzyl-3-hydroxypiperidine 2-(4-bromophenyl)-2-methylpropanoic acid Benzylacetoacetate Radiator Flux Zinc Chloride Solution (All Grades) Phenylazomalononitrile 2-Fluoro-6-iodobenzoic acid 3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 2,3-Difluoro-6-methoxybenzyl Chloride 2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Ethyl2-oxo-2,3,9,10-tetrahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(7H)-carboxylate Acetyl-meldrum's acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid N Ethylmethylamine Ethyl Methanesulfonate N N' DimethylEthylenediamine Lead II BromideRelated products of tetrahydrofuran
You may like
-
 Nickel Nitrate 98%View Details
Nickel Nitrate 98%View Details -
 NICKEL NITRATE 99%View Details
NICKEL NITRATE 99%View Details -
 Nickel(II) nitrate hexahydrate CAS 13478-00-7View Details
Nickel(II) nitrate hexahydrate CAS 13478-00-7View Details
13478-00-7 -
 Nickel(II) nitrate 99%View Details
Nickel(II) nitrate 99%View Details -
 Nickel(II) nitrate 98%View Details
Nickel(II) nitrate 98%View Details -
 Nickel nitrate hexahydrate 98%View Details
Nickel nitrate hexahydrate 98%View Details -
 Nickel(II) nitrate hexahydrate CAS 13478-00-7View Details
Nickel(II) nitrate hexahydrate CAS 13478-00-7View Details
13478-00-7 -
 Nickel(II) nitrate hexahydrate CAS 13478-00-7View Details
Nickel(II) nitrate hexahydrate CAS 13478-00-7View Details
13478-00-7
