NICKEL(II) CHLORIDE
Synonym(s):Nickel dichloride hydrate
- CAS NO.:69098-15-3
- Empirical Formula: Cl2H2NiO
- Molecular Weight: 147.61
- MDL number: MFCD00149808
- EINECS: 677-901-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
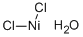
What is NICKEL(II) CHLORIDE?
The Uses of NICKEL(II) CHLORIDE
It is used in electroplating and as a catalyst for organic conversions, for example in chemo-selective thioacetalization of aldehydes. In combination with lithium aluminum hydride, it serves as a reducing agent for alkenes, alkynes, and organic halides; it can cleave N-O bond and open epoxides. It is a precursor to several nickel-phosphine complexes, such as bis(triphenylphosphine)nickel(II) chloride, which are used in alkyne trimerizations, carbonylations, and as catalysts in organic reactions such as Suzuki-Miyaura cross coupling reactions as an alternative to palladium(0) catalysts. It is the precursor to acetylacetonate complex of Ni, used for producing 1,5-cyclooctadiene complex, an important reagent in organonickel chemistry. It can be used to prepare the sandwich compound nickelocene through dimethoxyethane complex of nickel chloride.
Purification Methods
It crystallises from dilute HCl to form the green hexahydrate. At 70o this dehydrates to the tetrahydrate, and at higher temperatures it forms the anhydrous salt. It sublimes in yellow hexagonal scales in a stream of HCl. Store it in a desiccator as it is deliquescent. [Hart & Partington J Chem Soc 104 1943.]
Properties of NICKEL(II) CHLORIDE
| Melting point: | 1001 °C |
| vapor pressure | 1 mm Hg ( 671 °C) |
| form | Crystalline Aggregates |
| color | Green |
| Water Solubility | Freely soluble in water. Soluble in ethylene glycol, ethanol, and ammonium hydroxide. |
| Sensitive | Hygroscopic |
| Merck | 14,6505 |
| Exposure limits | NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 |
Safety information for NICKEL(II) CHLORIDE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H334:Sensitisation, respiratory H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for NICKEL(II) CHLORIDE
NICKEL(II) CHLORIDE manufacturer
Kronox Lab Sciences Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




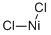
You may like
-
 Nickel(II) chloride hydrate 98%View Details
Nickel(II) chloride hydrate 98%View Details -
 Nickel(II) chloride hydrate CAS 69098-15-3View Details
Nickel(II) chloride hydrate CAS 69098-15-3View Details
69098-15-3 -
 Nickel(II) chloride hydrate CAS 69098-15-3View Details
Nickel(II) chloride hydrate CAS 69098-15-3View Details
69098-15-3 -
 Nickel(II) chloride hydrate CAS 69098-15-3View Details
Nickel(II) chloride hydrate CAS 69098-15-3View Details
69098-15-3 -
 Nickel(ii) chloride hydrate 95% CAS 69098-15-3View Details
Nickel(ii) chloride hydrate 95% CAS 69098-15-3View Details
69098-15-3 -
 Nickel(II) chloride hydrate CAS 69098-15-3View Details
Nickel(II) chloride hydrate CAS 69098-15-3View Details
69098-15-3 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1