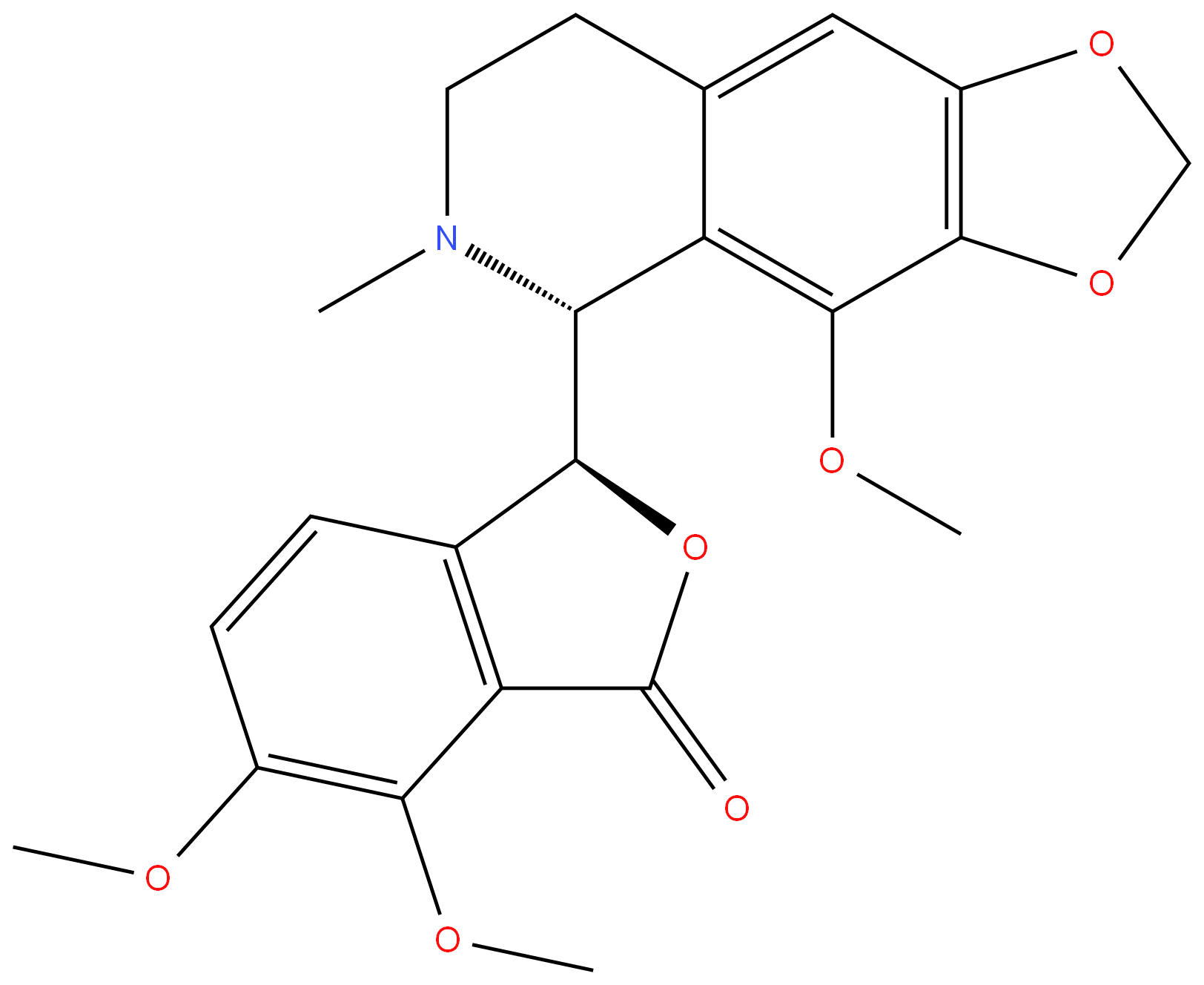
Narcotine
Synonym(s):(S,R)-Noscapine
- CAS NO.:128-62-1
- Empirical Formula: C22H23NO7
- Molecular Weight: 413.42
- MDL number: MFCD00069316
- EINECS: 204-899-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-17 18:22:24
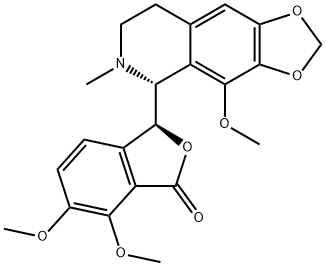
What is Narcotine?
Description
A major constituent of opium (Papaver sornni!erurn), this phthalideisolquinoline
base was probably first discovered by Derosne, but initially characterized by
Robiquet who gave it the formula C23H3S0 7N, changed to that now accepted by
Matthiesen and Foster. It crystallizes in colourless needles from EtOH and has
[α]D - 198° (c 1.0, CHC13); - 146° (c 2.0, toluene); - 147° (c 1.59, C6H6) and
+ 50° (1 % HCl). The alkaloid is a weak, monoacidic, tertiary base, forming
unstable salts with acids which are dissociated with H20. The hydrochloride
crystallizes with varying amounts of H20, is very soluble in water, readily
decomposing into basic salts on standing. The platinichloride is amorphous; the
oxalate has m.p. 174°C;[α]20D
+ 39.5° (H20); the phthalate, m.p. 160°C;
[α]22D + 115° (CHC13); sesquisulphate, colourless crystals of the hexahydrate and
the picrate, m.p. 1 74-S°C. The (+)-bromocamphorsulphonate has m.p. 110-
120°C; [α]D + 100.7° (CHC13) and the corresponding (-)-bromocamphorsul_x0002_phonate, m.p. l80.SoC; [α]D + 29° (CHC13). The alkaloid yields an N-oxide
which is a hygroscopic solid; [α]D + 135° (CHC13) furnishing a crystalline
hydrochloride, m.p. 193°C; platinichloride, m.p. 175°C and a picrate, m.p.
130°C.
The base is unstable to heat and when heated in a sealed tube yields a mixture
of dimethylnornarcotine, methylnornarcotine and nornarcotine. On heating with
H20 and 150°C, or on boiling with dilute acids, it is hydrolyzed to opianic acid
and hydrocotarnine. Similar degradations are brought about by acid oxidation orreduction, e.g. Zn and dilute HCl gives hydrocotarnine and meconin while dilute
HN03 yields cotarnine and opianic acid. Pharmacologically, narcotine resembles
thebaine (q.v.) in its action, being a reflex stimulant rather than a narcotic.
Chemical properties
Crystalline Solid
The Uses of Narcotine
Antitussive
The Uses of Narcotine
antiacne, antiproliferative agent
The Uses of Narcotine
Antitussive.
What are the applications of Application
Noscapine is a centrally acting antitussive agent
Indications
Investigated for use/treatment in lymphoma (non-hodgkin's), leukemia (lymphoid), cancer/tumors (unspecified), and multiple myeloma.
Definition
ChEBI: A benzylisoquinoline alkaloid that is 1,2,3,4-tetrahydroisoquinoline which is substituted by a 4,5-dimethoxy-3-oxo-1,3-dihydro-2-benzofuran-1-yl group at position 1, a methylenedioxy group at positions 6-7 and a methoxy group at position 8. Obtained from p ants of the Papaveraceae family, it lacks significant painkilling properties and is primarily used for its antitussive (cough-suppressing) effects.
brand name
Tusscapine (Fisons);Bequitussin;Bisolvon compositum;Broncha-tulisan eucalyptol;Broncho-tulisan eucalyptol;Brosolin-rectocap;Codipect;Codyl cum expectoras;Coscotab;Degoran;Dettuso;Difimetus compositum;Finipect;Hederix;Lyabex retard;Nipaxan;Nitepax;Nosaclin;Noscalin;Noscapect;Noscarex;Noscatuss;Reatos;Rectolmin bronquial;Ribelfan;Stilco;Teletux;Tucotin;Tuscapin;Tussamine plus;Tussanil n;Tusscalman;Tussicure;Tussisedal;Tussoretard.
World Health Organization (WHO)
Noscapine, a centrally-acting cough suppressant and one of several alkaloids present in papaveretum (opium concentrate) was introduced into medicine many years ago. Subsequently, it was shown to increase the number of chromosomes in mammalian cell lines maintained in vitro. Although the clinical significance of this finding is uncertain, restrictive action was taken in a few countries since the possibility of a genotoxic effect cannot be excluded. On 4 December 1992 the European Committee on Proprietary Medicinal Products concluded that the available evidence does not indicate that use of noscapine holds any significant hazard. The Swedish Medical Products Agency also concluded that there is no justification to restrict the use of noscapine in women of childbearing age.
Biological Functions
Noscapine is a naturally occurring product of the opium poppy. It is a benzylisoquinoline with no analgesic or other CNS effects. Its antitussive effects are weak, but it is used in combination with other agents in mixtures for cough relief.
Hazard
Narcotic, use legally restricted.
Safety Profile
Moderately toxic by ingestion andsubcutaneous routes. Mutation data reported. Anantitussive. When heated to decomposition it emits toxicfumes of NOx.
Metabolism
Not Available
References
Robiquet., Ann. Chim. Phys., 5,275 (1817)
Matthiessen, Foster.,!. Chern. Soc., 16,342 (1863)
Perkin, Robinson., ibid, 99,775 (1911)
Polonovski, Polonovski., Bull. Soc. Chim. Fr., 47, 361 (1930)
Lovell., Acta Cryst., 6, 869 (1953)
Barnes., Can. f. Chern., 33,444 (1955)
Safe, Moir., ibid, 160 (1964)
Stereochemistry:
Battersby, Spenser., Tetrahedron Lett., 11 (1964)
Properties of Narcotine
| Melting point: | 174-176 °C(lit.) |
| Boiling point: | 532.6°C (rough estimate) |
| alpha | -200 º (c=1 in chloroform) |
| Density | 1.395 |
| refractive index | 1.5614 (estimate) |
| storage temp. | -20°C Freezer, Under Inert Atmosphere |
| solubility | Practically insoluble in water, soluble in acetone, slightly soluble in ethanol (96 per cent). It dissolves in strong acids; on dilution of the solution with water, the base may be precipitated. |
| form | neat |
| pka | 7.8(at 25℃) |
| color | Orthorhombic prisms or stout needles from alc |
| optical activity | [α]20/D 200°, c = 1 in chloroform |
| Water Solubility | 302.9mg/L(25 ºC) |
| Merck | 13,6752 |
| NIST Chemistry Reference | Narcotine alkaloid(128-62-1) |
| EPA Substance Registry System | 1(3H)-Isobenzofuranone, 6,7-dimethoxy-3-[(5R)-5,6,7,8-tetrahydro-4-methoxy-6-methyl-1,3-dioxolo[4,5-g]isoquinolin-5-yl]-, (3S)- (128-62-1) |
Safety information for Narcotine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects |
Computed Descriptors for Narcotine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 ?128-62-1 Nor Noscapine 98%View Details
?128-62-1 Nor Noscapine 98%View Details
?128-62-1 -
 128-62-1 98%View Details
128-62-1 98%View Details
128-62-1 -
 Noscapine 99%View Details
Noscapine 99%View Details
128-62-1 -
 128-62-1 98%View Details
128-62-1 98%View Details
128-62-1 -
 Noscapine CAS 128-62-1View Details
Noscapine CAS 128-62-1View Details
128-62-1 -
 (S,R)-Noscapine CAS 128-62-1View Details
(S,R)-Noscapine CAS 128-62-1View Details
128-62-1 -
 Noscapine CAS 128-62-1View Details
Noscapine CAS 128-62-1View Details
128-62-1 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
