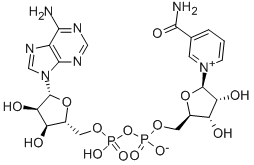
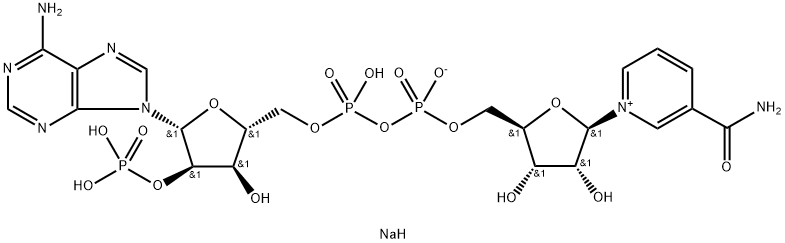
NADH, disodium salt
Synonym(s):NADH;β-NADH;β-DPNH;DPNH;Diphosphopyridine nucleotide reduced
- CAS NO.:606-68-8
- Empirical Formula: C21H27N7Na2O14P2
- Molecular Weight: 709.4
- MDL number: MFCD00036200
- EINECS: 210-123-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08

What is NADH, disodium salt?
Description
β-Nicotinamide adenine dinucleotide (NAD+) and β-Nicotinamide adenine dinucleotide, reduced (NADH) comprise a coenzyme redox pair (NAD+:NADH) involved in a wide range of enzyme catalyzed oxidation reduction reactions. In addition to its redox function, NAD+/NADH is a donor of ADP-ribose units in ADP-ribosylaton (ADP-ribosyltransferases; poly(ADP-ribose) polymerases ) reactions and a precursor of cyclic ADP-ribose (ADP-ribosyl cyclases).
As a reagent, NADH can be used in enzyme cycling assays to amplify detection of activity of biologically relevant enzymes or metabolites present in low concentrations.
Chemical properties
White to beige powder
The Uses of NADH, disodium salt
One of the biologically active forms of nicotinic acid. Serves as a coenzyme of hydrogenases and dehydrogenases. NAD usually acts as a hydrogen acceptor, forming NADH which then serves as a hydrogen d onor in the respiratory chain. Present in living cells primarily in the reduced form (NADPH) and is involved in synthetic reactions. Occurs in 2 forms, α-NAD and β-NAD, distinguished by the configura tion of the ribosyl nicotinamide linkage. Only the β-anomer is bioactive.
The Uses of NADH, disodium salt
One of the biologically active forms of nicotinic acid. Serves as a coenzyme of hydrogenases and dehydrogenases. NAD usually acts as a hydrogen acceptor, forming NADH which then serves as a hydrogen donor in the respiratory chain. Present in living cells primarily in the reduced form (NADPH) and is involved in synthetic reactions. Occurs in 2 forms, α-NAD and β-NAD, distinguished by the configuration of the ribosyl nicotinamide linkage. Only the β-anomer is bioactive.
The Uses of NADH, disodium salt
β-Nicotinamide adenine dinucleotide, reduced disodium salt has been used in the preparation of standard curve.
What are the applications of Application
β-Nicotinamide Adenine Dinucleotide Disodium Salt, reduced form is involved in a wide range of enzyme catalyzed oxidation reduction reactions
What are the applications of Application
NADH disodium salt is a coenzyme of oxidoreductases
What are the applications of Application
β-Nicotinamide adenine dinucleotide, reduced disodium salt is a regenerating electron donor coenzyme
Definition
NADH, disodium salt is a dehydrogenase complex that is the reduced formof NAD. As a reagent, NADH can be used in enzyme cycling assays to amplify detection of activity of biologically relevant enzymes or metabolites present in low concentrations.
Biological Functions
NADH disodium salt (Disodium NADH) is an orally active reduced coenzyme. NADH disodium salt is a donor of ADP-ribose units in ADP-ribosylaton reactions and a precursor of cyclic ADP-ribose. NADH disodium salt plays a role as a regenerative electron donor in cellular energy metabolism, including glycolysis, β-oxidation and the tricarboxylic acid (TCA) cycle.
General Description
β-Nicotinamide adenine dinucleotide (β-NAD) regulates energy metabolism and immunity. It is a cofactor for mitochondrial deacetylase sirtuin-3 enzyme and modulates inflammasome assembly. β-NAD supresses interleukin-1β levels in monocytic cells in inflammatory syndromes. β-NAD released by neurosecretory cells is a potential neurotransmitter. β-NAD is a vascular mediator in lung endothelial cells and may play a protective role against cytokine mediated inflammation.
Biochem/physiol Actions
NADH is a coenzyme that functions as a regenerating electron donor in catabolic processes including glycolysis, β-oxidation and the citric acid cycle (Krebs cycle, TCA cycle). It participates in cell signaling events as well, for example as a substrate for the poly (ADP-ribose) polymerases (PARPs) during the DNA damage response. The NAD+/NADH dependent sirtuins play key roles in stress responses during events involving energy metabolism, with implications in cancer biology, diabetes and neurodegenerative disease.
Biotechnological Applications
Reduced β-nicotinamide adenine dinucleotide (NADH) plays a major role in metabolism as a cofactor in redox reactions and as a mobile electron carrier. NADH is a high energy compound that donates electrons to the electron transport chain to provide energy for ATP production by oxidative phosphorylation. NADH is a required oxidizing cosubstrate in fermentation, which regenerates NAD. NADH is fluorescent, which provides for a relatively simple way to detect NADH in biological samples. NADH is also used in enzyme cycling assays to detect relevant biological molecules in tissues.
in vitro
NADH is unstable under acidic conditions but it is stable under alkaline conditions.
NADH (0-1 mM; 0-12 h) increases NAD levels in various mammalian cell lines+.
NADH (1 mM; 24 h) causes low toxicity and protects cells from genotoxicity.
in vivo
NADH (5 μmol/mouse; i.p.; once) increases urinary excretion of nicotinamide and its metabolites in mice.
NADH (500 mg/kg; i.g.; once) promotes alcohol metabolism and prevents or ameliorates early liver injury caused by acute alcohol exposure in ethanol-loaded mice.
NADH (1000 mg/kg; i.p.; once) enhances tissue NAD levels in male C57BL/6J mice+.
Purification Methods
This coenzyme is available in high purity, and it is advisable to buy a fresh preparation rather than to purify an old sample as purification will invariably lead to a more impure sample contaminated with the oxidised form (NAD). It has UV max at 340nm ( 6,200 M-1cm-1) at which wavelength the oxidised form NAD has no absorption. At 340nm a 0.161mM solution in a 1cm (pathlength) cell has an absorbance of 1.0 unit. The purity is best checked by the ratio A280nm/A340nm ~2.1, a value which increases as oxidation proceeds. The dry powder is stable indefinitely at -20o. Solutions in aqueous buffers at pH ~7 are stable for extended periods at -20o and for at least 8hours at 0o, but are oxidised more rapidly at 4o in a cold room (e.g. almost completely oxidised overnight at 4o). [UV: Drabkin J Biol Chem 175 563 1945, Fluorescence: Boyer & Thorell Acta Chem Scand 10 447 1956, Redox: Rodkey J Biol Chem 234 188 1959, Schlenk in The Enzymes 2 250, 268 1951, Kaplan in The Enzymes 3 105, 112 1960.] Deuterated NADH, i.e. NADD, has been purified through the anion exchange resin AG-1 x 8 (100-200 mesh, formate form) and through a Bio-Gel P-2 column. [Viola et al. Anal Biochem 96 334 1979, Beilstein 26 III/IV 3639.]
Definition
The NAD+ Is the oxidized form, that is, a state in which it loses an electron. NADH is a reduced form of the molecule, which means that it gains the electron lost by NAD+. Redox reactions involving electron transfers play a central role in energy creation. When NAD+ takes an electron from glucose, it becomes NADH. NADH transports this electron to mitochondria where the cell can take the energy that is stored in the electron. NADH then donates the electron to oxygen, converting it back to NAD+.
References
[2]. Kimura N, et al. Comparison of metabolic fates of nicotinamide, NAD+ and NADH administered orally and intraperitoneally; characterization of oral NADH. J Nutr Sci Vitaminol (Tokyo). 2006 Apr;52(2):142-8. [Content Brief]
[3]. Wu K, et al. NADH and NRH as potential dietary supplements or pharmacological agents for early liver injury caused by acute alcohol exposure. Journal of Functional Foods, 2021, 87: 104852.
Properties of NADH, disodium salt
| Melting point: | 140-142°C |
| Density | 1.955 at 20℃ |
| vapor pressure | 0.73Pa at 20-50℃ |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | H2O: 50 mg/mL, clear to nearly clear, yellow |
| form | Powder |
| color | Yellow |
| PH | 7.5 (100mg/mL in water, ±0.5) |
| Water Solubility | soluble |
| BRN | 5230241 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 606-68-8 |
| EPA Substance Registry System | Reduced .beta.-nicotinamide adenine dinucleotide disodium salt (606-68-8) |
Safety information for NADH, disodium salt
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for NADH, disodium salt
| InChIKey | QRGNQKGQENGQSE-WUEGHLCSSA-L |
NADH, disodium salt manufacturer
Innovative
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 NADH, disodium salt CAS 606-68-8View Details
NADH, disodium salt CAS 606-68-8View Details
606-68-8 -
 Beta-nicotinamide adenine dinucleotide disodium salt hydrate CAS 606-68-8View Details
Beta-nicotinamide adenine dinucleotide disodium salt hydrate CAS 606-68-8View Details
606-68-8 -
 ß-Nicotinamide Adenine Dinucleotide Disodium Salt (Reduced) (ß-NADH.Na2 CAS 606-68-8View Details
ß-Nicotinamide Adenine Dinucleotide Disodium Salt (Reduced) (ß-NADH.Na2 CAS 606-68-8View Details
606-68-8 -
![β-Nicotinamide Adenine Dinucleotide Disodium Salt, reduced form [for Biochemical Research] CAS 606-68-8](https://img.chemicalbook.in//Content/image/CP5.jpg) β-Nicotinamide Adenine Dinucleotide Disodium Salt, reduced form [for Biochemical Research] CAS 606-68-8View Details
β-Nicotinamide Adenine Dinucleotide Disodium Salt, reduced form [for Biochemical Research] CAS 606-68-8View Details
606-68-8 -
 Dihydronicotinamide adenine dinucleotide disodium salt CAS 606-68-8View Details
Dihydronicotinamide adenine dinucleotide disodium salt CAS 606-68-8View Details
606-68-8 -
 NICOTINAMIDE ADENINE DINUCLEOTIDE REDUCED DISODIUM For Biochemistry CAS 606-68-8View Details
NICOTINAMIDE ADENINE DINUCLEOTIDE REDUCED DISODIUM For Biochemistry CAS 606-68-8View Details
606-68-8 -
 NADH CAS 606-68-8View Details
NADH CAS 606-68-8View Details
606-68-8 -
 Beta-Nicotinamide Adenine Dinucleotide, Reduced Disodium Salt (Beta-NADH) (CAS Number: 606-68-8)View Details
Beta-Nicotinamide Adenine Dinucleotide, Reduced Disodium Salt (Beta-NADH) (CAS Number: 606-68-8)View Details
606-68-8
