N-METHYLBUTYLAMINE
Synonym(s):N-Butylmethylamine;N-Methylbutylamine
- CAS NO.:110-68-9
- Empirical Formula: C5H13N
- Molecular Weight: 87.16
- MDL number: MFCD00009426
- EINECS: 203-791-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
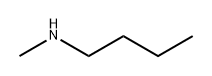
What is N-METHYLBUTYLAMINE?
Chemical properties
CLEAR COLOURLESS LIQUID
Chemical properties
Butyl amines are highly flammable, colorless liquids (n-turns yellow on standing) with ammoniacal or fishlike odors. n-isomer:
The Uses of N-METHYLBUTYLAMINE
Intermediate.
What are the applications of Application
N-Butylmethylamine is used in synthesis of N-n-butyl-N-methyl-N-2-(1-phenyl)propyl-amine and 16α-(bromoalkylamide) derivatives of estradiol.
Definition
ChEBI: A secondary aliphatic amine having methyl and n-butyl as the two alkyl groups.
General Description
A water-white liquid with an ammonia-like odor. Density 0.736 g / cm3 and flash point 35°F (Aldrich) .Vapors heavier than air. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
N-METHYLBUTYLAMINE neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Hazard
Flammable, dangerous fire risk.
Health Hazard
May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion, skin contact, and intraperitoneal routes. Mildly toxic by inhalation. A skin and severe eye irritant. Flammable liquid when exposed to heat, sparks, or flame. To fight fire, use alcohol foam. When heated to decomposition it emits toxic fumes of NOx. See also AMINES.
Potential Exposure
Alert: (n-isomer): Possible risk of forming tumors, suspected of causing genetic defects, suspected reprotoxic hazard, Primary irritant (w/o allergic reaction), (sec-isomer): Drug. n-Butylamine is used in pharmaceuticals; dyestuffs, rubber, chemicals, emulsifying agents; photography, desizing agents for textiles; pesticides, and synthetic agents. sec-Butylamine is used as a fungistate. tert-Butylamine is used as a chemical intermediate in the production of tert-Butylaminoethyl methacrylate (a lube oil additive); as an intermediate in the production of rubber and in rust preventatives and emulsion deterrents in petroleum products. It is used in the manufacture of several drugs
Shipping
UN1125 n-Butylamine, Hazard Class: 3; Labels: 3—Flammable liquid, 8—Corrosive material. UN2014 Isobutylamine, Hazard Class: 3; Labels: 3—Flammable liquid, 8—Corrosive material
Incompatibilities
May form explosive mixture with air. May accumulate static electrical charges, and may causeignition of its vapors. n-Butylamine is a weak base; reacts with strong oxidizers and acids, causing fire and explosion hazard. Incompatible with organic anhydrides; isocyanates, vinyl acetate; acrylates, substituted allyls; alkylene oxides; epichlorohydrin, ketones, aldehydes, alcohols, glycols, phenols, cresols, caprolactum solution. Attacks some metals in presence of moisture. The tert-isomer will attack some forms of plastics
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner andscrubber. All federal, state, and local environmental regulations must be observed.
Properties of N-METHYLBUTYLAMINE
| Melting point: | -75 °C |
| Boiling point: | 90.5-91.5 °C(lit.) |
| Density | 0.736 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 35 °F |
| storage temp. | Store below +30°C. |
| form | clear liquid |
| pka | pK1: 10.90(+1) (25°C) |
| color | Colorless to Light orange to Yellow |
| explosive limit | 1.3%(V) |
| BRN | 1209231 |
| CAS DataBase Reference | 110-68-9(CAS DataBase Reference) |
| EPA Substance Registry System | 1-Butanamine, N-methyl- (110-68-9) |
Safety information for N-METHYLBUTYLAMINE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H302:Acute toxicity,oral H311:Acute toxicity,dermal H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for N-METHYLBUTYLAMINE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran

You may like
-
 N-Methylbutylamine CAS 110-68-9View Details
N-Methylbutylamine CAS 110-68-9View Details
110-68-9 -
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 Cyaclopentane carboxylic acid 99%View Details
Cyaclopentane carboxylic acid 99%View Details
3400-45-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0