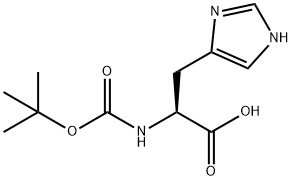
Boc-His(Trt)-OH
Synonym(s):Nα-Boc-N(im)-trityl-L -histidine;Boc-His(Trt)-OH;N-α-t.-Boc-N-im-trityl-L-histidine
- CAS NO.:32926-43-5
- Empirical Formula: C30H31N3O4
- Molecular Weight: 497.58
- MDL number: MFCD00153307
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-23 19:22:53
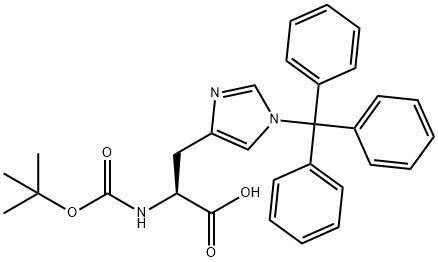
What is Boc-His(Trt)-OH?
Chemical properties
White to off white powder
The Uses of Boc-His(Trt)-OH
Boc-His(Trt)-OH was used in the study to prepare and study structure-activity relationship of amino acid amides as selective inhibitors for dipeptidyl peptidases.
The Uses of Boc-His(Trt)-OH
Boc-His(Trt)-OH is a histidine derivative. Its a protected histidine derivative for only the critical coupling step. After coupling the Boc- and Trt-groups are cleaved simultaneously by TFA.
Preparation
The benzyl 2-tert-butoxycarbonylamino-3-(1-trimethylimidazol-4-yl)propionate was dissolved in methanol (20 mL), and potassium hydroxide was added to the reaction system. The reaction system was stirred for 1 h after THF was added to the reaction system. The solvent is removed under vacuum depressurization, and the mixture is then extracted with water and Et2O. Separate the two phases and acidify the aqueous layer with dilute HCl, extract it with Et2O, combine all the ether organic layer and wash it with water and saline, dry the organic layer with anhydrous magnesium sulfate, filter to remove the solvent, and concentrate the filtrate under vacuum under reduced pressure to obtain Boc-His(Trt)-OH.
What are the applications of Application
N-Boc-N'-Trityl-L-histidine (Boc-His(Trt)-OH) can be used as an intermediate in biochemistry and medicinal chemistry and can be used in the synthesis of peptide drug molecules, vitamins, and molecules with specific biologically active functions. In organic synthesis transformation, the carboxyl group in N-Boc-N'-trityl-L-histidine can undergo corresponding condensation reactions with amine compounds to obtain the corresponding amide products; in addition, in dithionyl chloride Under the action of N-Boc-N'-trityl-L-histidine, intramolecular dehydration cyclization reaction can be performed to obtain cyclic amide derivatives.
reaction suitability
Reaction type: Boc solid-phase peptide synthesis
Properties of Boc-His(Trt)-OH
| Melting point: | ~130 °C (dec.) |
| Boiling point: | 667.5±55.0 °C(Predicted) |
| alpha | 12.5 º (C=1% IN MEOH) |
| Density | 1.16±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Sparingly) |
| form | Solid |
| pka | 3.17±0.10(Predicted) |
| color | White to Off-White |
| optical activity | [α]20/D +12.5±1.0°, c = 1% in methanol |
| BRN | 732035 |
| CAS DataBase Reference | 32926-43-5(CAS DataBase Reference) |
Safety information for Boc-His(Trt)-OH
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Boc-His(Trt)-OH
| InChIKey | OYXZPXVCRAAKCM-SANMLTNESA-N |
| SMILES | C(O)(=O)[C@H](CC1N=CN(C(C2=CC=CC=C2)(C2=CC=CC=C2)C2=CC=CC=C2)C=1)NC(OC(C)(C)C)=O |
Boc-His(Trt)-OH manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![(-)-2-[METHYLAMINO]-1-PHENYLPROPANE](https://img.chemicalbook.in/CAS/GIF/33817-09-3.gif)
You may like
-
 Boc-His(Trt)-OH 98% (HPLC) CAS 32926-43-5View Details
Boc-His(Trt)-OH 98% (HPLC) CAS 32926-43-5View Details
32926-43-5 -
 Boc-His(Trt)-OH, ≥98% (TLC) CAS 32926-43-5View Details
Boc-His(Trt)-OH, ≥98% (TLC) CAS 32926-43-5View Details
32926-43-5 -
 N-Boc-N-trityl-L-histidine CAS 32926-43-5View Details
N-Boc-N-trityl-L-histidine CAS 32926-43-5View Details
32926-43-5 -
 Boc-His(Trt)-OH CAS 32926-43-5View Details
Boc-His(Trt)-OH CAS 32926-43-5View Details
32926-43-5 -
 Boc-His(Trt)-OH CAS 32926-43-5View Details
Boc-His(Trt)-OH CAS 32926-43-5View Details
32926-43-5 -
 32926-43-5 N-Boc-N'-trityl-L-histidine 98%View Details
32926-43-5 N-Boc-N'-trityl-L-histidine 98%View Details
32926-43-5 -
 32926-43-5 99%View Details
32926-43-5 99%View Details
32926-43-5 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1