N-BOC-1,6-diaminohexane
Synonym(s):N-Boc-1,6-diaminohexane;tert-Butyl-N-(6-aminohexyl)carbamate
- CAS NO.:51857-17-1
- Empirical Formula: C11H24N2O2
- Molecular Weight: 216.32
- MDL number: MFCD00671489
- EINECS: 628-984-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
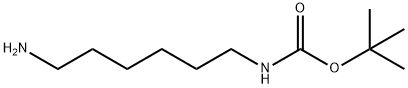
What is N-BOC-1,6-diaminohexane?
Chemical properties
Off-White Solid
The Uses of N-BOC-1,6-diaminohexane
N-Boc-1,6-diaminohexane is used to prepare 1,3-Bis[6-(Boc-amino)hexyl]urea by reacting with carbonyl dichloride in the presence of triethylamine. Further, it is used as a reagent for the introduction of a C6-spacer.
What are the applications of Application
N-Boc-1,6-hexanediamine can be used as a linear hexyl spacer (C6-spacer) to synthesize:
Biodegradable poly(disulfide amine)s for gene delivery.
A multifunctional dendrimer for theranostics.
Polyamide platinum anti-cancer complexes designed to target cancer specific DNA sequences.
Self-assembled monolayers (SAMs) that resist adsorption of proteins.
[N-(6-(4-Hydroxy-6-isopropylamino-1,3,5-triazin-2-ylamino)hexyl)-5-hydroxy-1,4-naphthoquinone-3-propionamide] (JUG-HATZ), which can be used in designing electrochemical immunosensors.
Preparation
synthesis of N-BOC-1,6-diaminohexane: 1,6-Diaminohexane 3 (Fig. 1) (100 g, 0.86 mol) was dissolved in CH2Cl2 (300 ml) and cooled in an ice bath to 0–3°C. To the stirred solution 0.3 eq. di-tert-butyl bicarbonate (62.6 g, 0.29 mol) was added slowly over a period of 1 h. The reaction was allowed to warm up to r.t., and proceeded overnight. The reaction mixture was extracted with saturated aqueous NaHCO3 (50 ml, three times). The organic phases were pooled, dried, and evaporated under reduced pressure. The resulting oil was dissolved in 200 ml, 1 N HCl and extracted with ether. The aqueous phase was washed with ether, made basic to a pH of 10 with aqueous 2 N NaO3, and extracted with ethyl acetate. The organic phases were pooled, dried, and evaporated under reduced pressure. The resulting oil was dissolved in 200 ml, 1 N HCL and extracted with ether. The aqueous phase was washed with ether, made basic to a pH of 10 with aqueous 2 N NaOH, and extracted with ethyl acetate. The organic extracts were pooled, dried over Na2SO4 and evaporated to give 10.5 g of homogeneous N-BOC-1, 6- diaminohexane 2c (Fig. 1) as a yellow oil which was used without further purification.
TLC Rf 0.51; 1H NMR (CDCL3) d 4.54 (bs, 1H; NH), 3.05 1 2.62 (m, 4H; NCH2), 1.41 (m, 9H; CH3), 1.29 (m, 8H; CH2).
Evaluation of aminoalkylmethacrylate nanoparticles as colloidal drug carrier systems. Part I: synthesis of monomers, dependence of the physical properties on the polymerization methods
Properties of N-BOC-1,6-diaminohexane
| Boiling point: | 106-110 °C (0.3 mmHg) |
| Density | 0.965 g/mL at 20 °C(lit.) |
| refractive index | n |
| Flash point: | 125 °C |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Soluble in dichloromethane and ethyl acetate. |
| form | Viscous Liquid |
| pka | 12.93±0.46(Predicted) |
| color | Clear light yellow |
| Sensitive | Air Sensitive |
| BRN | 2089264 |
| CAS DataBase Reference | 51857-17-1(CAS DataBase Reference) |
Safety information for N-BOC-1,6-diaminohexane
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for N-BOC-1,6-diaminohexane
| InChIKey | RVZPDKXEHIRFPM-UHFFFAOYSA-N |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran



You may like
-
 N-Boc-1,6-diaminohexane CAS 51857-17-1View Details
N-Boc-1,6-diaminohexane CAS 51857-17-1View Details
51857-17-1 -
 N-(tert-Butoxycarbonyl)-1,6-diaminohexane CAS 51857-17-1View Details
N-(tert-Butoxycarbonyl)-1,6-diaminohexane CAS 51857-17-1View Details
51857-17-1 -
 N-Boc-1,6-Diaminohexane 99% (GC) CAS 51857-17-1View Details
N-Boc-1,6-Diaminohexane 99% (GC) CAS 51857-17-1View Details
51857-17-1 -
 N-Boc-1,6-diaminohexane 97% CAS 51857-17-1View Details
N-Boc-1,6-diaminohexane 97% CAS 51857-17-1View Details
51857-17-1 -
 N-Boc-1,6-hexanediamine CAS 51857-17-1View Details
N-Boc-1,6-hexanediamine CAS 51857-17-1View Details
51857-17-1 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
