N-Acetylneuraminic acid
Synonym(s):NAN;Sialic acid;NANA;Lactaminic acid;NANA, Lactaminic Acid, Sialic Acid
- CAS NO.:131-48-6
- Empirical Formula: C11H19NO9
- Molecular Weight: 309.27
- MDL number: MFCD00006620
- EINECS: 205-023-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
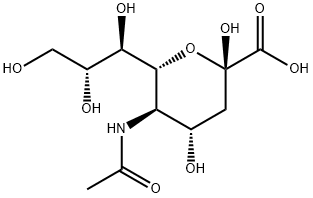
What is N-Acetylneuraminic acid?
Description
N-acetylneuraminic acid is an N-acyl derivative of neuraminic or acid amino sugar derivative, derived from N-acetylmannosamine and pyruvic acid. It is an important constituent of glycoproteins and glycolipids. N-acetylneuraminic acid occurs in many polysaccharides, glycoproteins, and glycolipids in animals and bacteria.
Physical properties
The numbering of the sialic acid structure begins at the carboxylate carbon and continues around the chain. The configuration which places the carboxylate in the axial position is the alpha-anomer.
The alpha-anomer is the form that is found when sialic acid is bound to glycans, however, in solution it is mainly (over 90 %) in the beta-anomeric form. A bacterial enzyme with sialic acid mutarotase activity, NanM, has been discovered which is able to rapidly equilibrate solutions of sialic acid to the resting equilibrium position of around 90 % beta 10 % alpha.
The Uses of N-Acetylneuraminic acid
N-Acetylneuraminic acid is an integral part of sialic acids (SAs), important functional sugars that play an important role in maintaining and improving brain health, detoxification, antibacterial, and immune enhancement. n-Acetylneuraminic acid is biologically useful for neurotransmission, leukocyte extravasation, viral or bacterial infection, and carbohydrate-protein recognition. n- Acetylneuraminic acid is used to study its biochemistry, metabolism and absorption in vivo and in vitro.
What are the applications of Application
N-Acetylneuraminic acid is the predominant mammalian sialic acid, mostly abundant in bacteria as well as in eukaryotic cells.
Definition
ChEBI: N-Acetylneuraminic acid is n-Acetylneuraminic acid with beta configuration at the anomeric centre. It has a role as an epitope. It is functionally related to a beta-neuraminic acid. It is a conjugate acid of a N-acetyl-beta-neuraminate.
Biosynthesis
In bacterial systems, sialic acids are biosynthesized by an aldolase enzyme. The enzyme uses a mannose derivative as a substrate, inserting three carbons from pyruvate into the resulting sialic acid structure. These enzymes can be used for chemoenzymatic synthesis of sialic acid derivatives.
Biological Functions
Sialic acid-rich glycoproteins (sialoglycoproteins) bind selectin in humans and other organisms. Metastatic cancer cells often express a high density of sialic acid-rich glycoproteins. This overexpression of sialic acid on surfaces creates a negative charge on cell membranes. This creates repulsion between cells (cell opposition) and helps these late-stage cancer cells enter the blood stream.
Sialic acid also plays an important role in human influenza infections.
Many bacteria also use sialic acid in their biology, although this is usually limited to bacteria that live in association with higher animals (deuterostomes). Many of these incorporate sialic acid into cell surface features like their lipopolysaccharide and capsule, which helps them evade the innate immune response of the host.[6] Other bacteria simply use sialic acid as a good nutrient source, as it contains both carbon and nitrogen and can be converted to fructose-6- phosphate, which can then enter central metabolism.
Sialic acid-rich oligo saccharides on the glyco conjugates ( glyco lipids, glyco proteins, proteoglycans) found on surface membranes help keep water at the surface of cells . The sialic acid - rich regions contribute to creating a negative charge on the cells' surfaces. Since water is a polar molecule with partial positive charges on both hydrogen atoms, it is attracted to cell surfaces and membranes. This also contributes to cellular fluid uptake.
Sialic acid can "hide" mannose antigens on the surface of host cells or bacteria from mannose - binding lectin . This prevents activation of complement.
Sialic acid in the form of poly sialic acid is an unusual posttranslational modification that occurs on the neural cell adhesion molecules (NCAMs). In the synapse, the strong negative charge of the polysialic acid prevents NCAM cross-linking of cells.
Biochem/physiol Actions
Both sialic acid and neuraminic acid are loosely used to refer to conjugates of neuraminic acid. N-Acetylneuraminic acid is often found as the terminal sugar of cell surface glycoproteins. Cell surface glycoproteins have important roles in cell recognition and interaction as well as in cell adhesion. Membrane glycoproteins are also important in tumor growth and metastases.
Properties of N-Acetylneuraminic acid
| Melting point: | 184-186 °C |
| Boiling point: | 449.56°C (rough estimate) |
| alpha | -32 º (c=2,water) |
| Density | 1.3580 (rough estimate) |
| refractive index | -32 ° (C=1, H2O) |
| storage temp. | -20°C |
| solubility | 50 g/L (20°C) |
| form | synthetic, crystalline |
| pka | 2.41±0.54(Predicted) |
| color | White to Off-White |
| Water Solubility | 50 g/L (20 ºC) |
| Sensitive | Air Sensitive |
| Merck | 14,8484 |
| BRN | 1716283 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 131-48-6(CAS DataBase Reference) |
| EPA Substance Registry System | Neuraminic acid, N-acetyl- (131-48-6) |
Safety information for N-Acetylneuraminic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for N-Acetylneuraminic acid
| InChIKey | SQVRNKJHWKZAKO-PFQGKNLYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran






You may like
-
 N-Acetylneuraminic Acid CAS 131-48-6View Details
N-Acetylneuraminic Acid CAS 131-48-6View Details
131-48-6 -
 N-Acetylneuraminic Acid extrapure (NANA) CAS 131-48-6View Details
N-Acetylneuraminic Acid extrapure (NANA) CAS 131-48-6View Details
131-48-6 -
 n-Acetylneuraminic acid CAS 131-48-6View Details
n-Acetylneuraminic acid CAS 131-48-6View Details
131-48-6 -
 N-Acetylneuramic acid 96% CAS 131-48-6View Details
N-Acetylneuramic acid 96% CAS 131-48-6View Details
131-48-6 -
 N-Acetylneuraminic acid 98% (HPLC) CAS 131-48-6View Details
N-Acetylneuraminic acid 98% (HPLC) CAS 131-48-6View Details
131-48-6 -
 N-(-)-Acetylneuraminic acid CAS 131-48-6View Details
N-(-)-Acetylneuraminic acid CAS 131-48-6View Details
131-48-6 -
 N-Acetylneuraminic acid hydrate 97% CAS 131-48-6View Details
N-Acetylneuraminic acid hydrate 97% CAS 131-48-6View Details
131-48-6 -
 N-Acetylneuraminic Acid, Synthetic CAS 131-48-6View Details
N-Acetylneuraminic Acid, Synthetic CAS 131-48-6View Details
131-48-6
