Amuvatinib
- CAS NO.:850879-09-3
- Empirical Formula: C23H21N5O3S
- Molecular Weight: 447.51
- MDL number: MFCD16038298
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-17 16:00:36
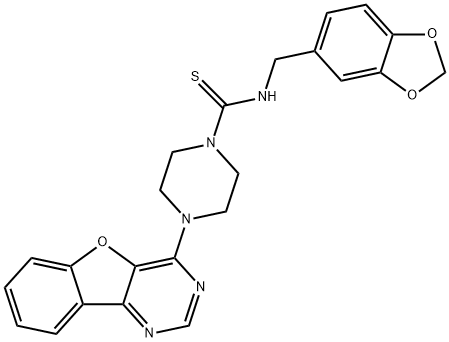
What is Amuvatinib?
Description
Amuvatinib is a multi-targeted inhibitor of receptor tyrosine kinases that inhibits c-Kit, platelet-derived growth factor receptor α (PDGFRα), and c-Met (IC50s = 10, 40, and 81 nM, respectively). It inhibits growth and induces apoptosis in prostate cancer cell lines, with additive effects achieved when combined with erlotinib . Amuvatinib sensitizes cancer cells to radiation and chemotherapeutic compounds, in part by inhibiting homologous recombination.
The Uses of Amuvatinib
MP-470 (Amuvatinib) is a potent and multi-targeted inhibitor of c-KitD816H, PDGFαV561D and Flt3D835Y with IC50 of 10 nM, 40 nM and 81 nM, respectively.
What are the applications of Application
Amuvatinib is a multi-targeted tyrosine kinase inhibitor of PDGF and c-Kit
Definition
ChEBI: N-(1,3-benzodioxol-5-ylmethyl)-4-(4-benzofuro[3,2-d]pyrimidinyl)-1-piperazinecarbothioamide is a N-arylpiperazine.
Clinical Use
Amivantamab is the first drug approved by the FDA for the treatment of patients with NSCLC and EGFR exon 20 insertion mutations.
Mode of action
Μechanisticly, amuvatinib inhibits tyrosine kinase receptor KIT through occupying its ATP binding domain (IC50 < 0.1 μM) and disrupts DNA repair through suppression of homologous recombination protein Rad51 as well as synergistic effects in combination with double stranded DNA damaging agents.
References
1. Effects of combining amuvatinib (MP-470) with DNA-damaging agents in osteosarcoma cell lines
2. Janssen submits supplemental biologics license application to the U.S. Food and Drug Administration seeking approval of RYBREVANT? (amivantamab-vmjw) in combination with chemotherapy for the first-line treatment of patients with locally advanced or metastatic EGFR exon 20 insertion mutation-positive non-small cell lung cancer. News release.
3. Raoul Tibes, Gil Fine, Gavin Choy, Sanjeev Redkar, Pietro Taverna, Aram Oganesian, Amarpao Sahai, Mohammad Azab and Anthony W. Tolcher. A phase I, first-in-human dose-escalation study of amuvatinib, a multi-targeted tyrosine kinase inhibitor, in patients with advanced solid tumors. Cancer Chemother Pharmacol (2013) 71: 463-471
4. Gavin Choy, Rajashree Joshi-Hangal, Aram Oganesian, Gil Fine, Scott Rasmussen, Joanne Collier, James Kissling, Amarpal Sahai, Mohammad Azab and Sanjeev Redkar. Saftety, tolerability, and pharmacokinetics of amuvatinib from three phase 1 clinical studies in healthy volunteers. Cancer Chemother Pharmacol (2012) 70: 183-190
Properties of Amuvatinib
| Density | 1.443 |
| storage temp. | Store at -20°C |
| solubility | ≥22.4 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
| form | solid |
| color | White to off-white |
| CAS DataBase Reference | 850879-09-3 |
Safety information for Amuvatinib
Computed Descriptors for Amuvatinib
New Products
1-Amino-1-cyclohexanecarboxylic acid 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione S-Methylisothiosemicarbazide hydroiodide ELECTROLYTIC IRON POWDER 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate Decanonitrile N,N'-diallyl-1,3-diaminopropanedihydrochloride N-(3-Nitrophenyl)cyclopropanecarboxamide (2-amino-2-phenylethyl)(methyl)amine Methyl-2-acetamidobenzoate methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 4-cyano benzaldehyde 2-hydroxy-5-bromo pyridine 5-Fluoro-2-Oxindole 3,5-Dichloro-2,6-Dimethyl-1h-Pyridin-4-One (9H-fluoren-9-yl)methyl carbonochloridate 2-methyl-5-nitroaniline (S)-1-(tert-butoxycarbonyl)-4-oxopyrrolidine-2-carboxylic acidRelated products of tetrahydrofuran
You may like
-
 Amuvatinib 95% CAS 850879-09-3View Details
Amuvatinib 95% CAS 850879-09-3View Details
850879-09-3 -
 Amuvatinib >95% CAS 850879-09-3View Details
Amuvatinib >95% CAS 850879-09-3View Details
850879-09-3 -
 4 Chloro 6,7 Dimethoxy Quinoline (Cabozantinib intermediate)View Details
4 Chloro 6,7 Dimethoxy Quinoline (Cabozantinib intermediate)View Details
35654-56-9 -
 3,4 Difluoro benzaldehydeView Details
3,4 Difluoro benzaldehydeView Details
34036-07-2 -
 10,10 Dimethyl AnthroneView Details
10,10 Dimethyl AnthroneView Details
5447-86-9 -
 Methyl 3 cyclohexene 1 carboxylateView Details
Methyl 3 cyclohexene 1 carboxylateView Details
6493-77-2 -
 Indazole 3 carboxylic acidView Details
Indazole 3 carboxylic acidView Details
4498-67-3 -
 2 Hydroxy 4 methoxy benzoic acidView Details
2 Hydroxy 4 methoxy benzoic acidView Details
2237-36-7