Myrrh Oil
- CAS NO.:8016-37-3
- Molecular Weight: 0
- MDL number: MFCD00240996
- EINECS: 616-981-9
- Update Date: 2024-12-18 14:08:57
What is Myrrh Oil?
Description
Refer to MYRRH, GUM.
Chemical properties
Myrrh oil is obtained from the gum by steam distillation; it is a light brown or
green liquid with the characteristic odor of the gum.
d2525 0.995–1.014; n20D 1.5190–1.5275; α20D ?83 ° to ?60 °; acid number: 2–13;
saponification number: 9–35; solubility: 1 vol in 7–10 vol of 90% ethanol; solutions
are occasionally opalescent or turbid.
Typical aroma-determining compounds of themyrrh plant are furanosesquiterpenoids
such as (?)-lindestrene. Myrrh oil and myrrh
resinoids are used in perfume compositions to create oriental notes.Myrrh extract
and Myrrh tincture are also utilized in oral care and corresponding preparations.
Chemical properties
The oil, obtained by steam distillation of the gum, in approximately 3 to 8% yields. It has a pungent, balsamic, warm odor and corresponding flavor. The oil tends to darken and thicken on exposure to air and light.
Physical properties
The oil is a light-brown to green liquid. It is soluble in most fixed oils, but is only slightly soluble in mineral oil. It is insoluble in glycerin and in propylene glycol. Under the influence of air and light, the oil becomes darker in color and more viscous
Occurrence
Found in several species of gum-resin Commiphora (fam. Burseraceae), mainly C. myrrha, C. abyssinica and C. schiniperi.
The Uses of Myrrh Oil
Myrrh traditionally has been used internally to treat upper respiratory congestion, pharyngitis, gingivitis, mouth ulcers, stomatitis, leprosy, syphilis, and leg ulcers. Topically, it is used to treat wounds, decubitus ulcers, and hemorrhoids. Contemporary use is mostly limited to fl avoring in foods and fragrance in cosmetic products.
Preparation
By steam distillation of the gum.
Definition
Extractives and their physically modified derivatives. Commiphora, Burseraceae.
Essential oil composition
Main constituents include d-pinene, dipentene, limonene, cinnamaldehyde, cuminaldehyde, eugenol, m-cresol, sesquiterpenes and formic and acetic acids.
Properties of Myrrh Oil
| Boiling point: | 220 °C(lit.) |
| Density | 1.003 g/mL at 25 °C(lit.) |
| refractive index | n |
| FEMA | 2766 | MYRRH OIL (COMMIPHORA SPP.) |
| Flash point: | >230 °F |
| Odor | at 100.00 %. balsamic woody musty amber spicy toffee gourmand |
| EPA Substance Registry System | Oils, myrrh (8016-37-3) |
Safety information for Myrrh Oil
Computed Descriptors for Myrrh Oil
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
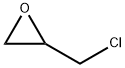
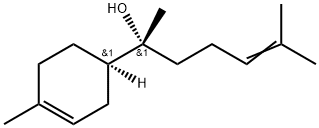



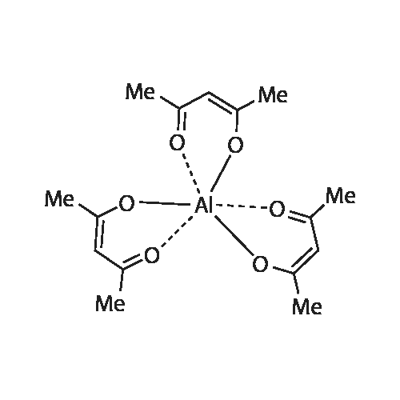


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1