529-44-2
Product Name:
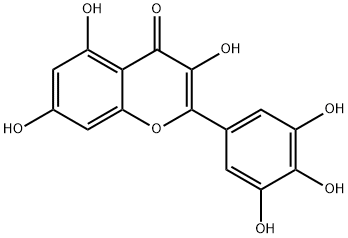
Myricetin
Formula:
C15H10O8
Synonyms:
3,3ʹ,4ʹ,5,5ʹ,7-Hexahydroxyflavone, Hsp70 Inhibitor II;3,3′,4′,5,5′,7-Hexahydroxyflavone;Cannabiscetin;Myricetin - CAS 529-44-2 - Calbiochem;Myricetol
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Yellow needles from dilute alcohol |
| Melting Point | 357 °C |
| Solubility | Sparingly soluble in boiling water; soluble in alcohol. Practically insoluble in chloroform, acetic acid |
| Decomposition | When heated to decomposition it emits acrid smoke and irritating vapors. |
| Collision Cross Section | 174.4 Ų [M+H]+ [CCS Type: DT, Method: stepped-field] |
| Other Experimental Properties | Crystals, mp 213 °C /Hexaacetate/ |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 318.23 g/mol |
|---|---|
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 1 |
| Exact Mass | 318.03756727 g/mol |
| Monoisotopic Mass | 318.03756727 g/mol |
| Topological Polar Surface Area | 148 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 506 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Myricetin is a hexahydroxyflavone that is flavone substituted by hydroxy groups at positions 3, 3', 4', 5, 5' and 7. It has been isolated from the leaves of Myrica rubra and other plants. It has a role as a cyclooxygenase 1 inhibitor, an antineoplastic agent, an antioxidant, a plant metabolite, a food component, a hypoglycemic agent and a geroprotector. It is a hexahydroxyflavone and a 7-hydroxyflavonol. It is a conjugate acid of a myricetin(1-).
