MORACIZINE HYDROCHLORIDE
- CAS NO.:29560-58-5
- Empirical Formula: C22H26ClN3O4S
- Molecular Weight: 463.98
- MDL number: MFCD00329917
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
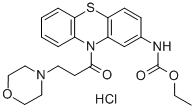
What is MORACIZINE HYDROCHLORIDE?
Description
Moricizine hydrochloride is a phenothiazine derivative with antiarrhythmic activity developed by the Soviet Academy of Medical Sciences. It is licensed to Dupont-Sanko in a joint venture. Moricizine hydrochloride is the first drug licensed to a U.S. company by the Soviet Union and was introduced by Dupont in the U.S. in 1990 for specific use in Iife-threatening ventricular arrhythmias. This narrow indication reflects its proarrhythmic potential. The incidence of this adverse reaction, however, is lower than that of other antiarrhythmic agents.
Originator
Acad. Med. Sci. (USSR)
The Uses of MORACIZINE HYDROCHLORIDE
Cardiac depressant (anti-arrhythmic).
The Uses of MORACIZINE HYDROCHLORIDE
Moricizine Hydrochloride is a Phenothiazine (P318040) derivative, which It was used as an antiarrhythmic agent. It is also shown that moracizine is effective in suppressing premature ventricular contractions, couplets, and nonsustained ventricular tachycardia.
Definition
ChEBI: A hydrochloride salt obtained from equimola amounts of moricizine and hydrogen chloride.
Manufacturing Process
To a solution of 10 g (0.035 mole) of ethyl phenthiazine-2-carbamate in 30 ml
of anhydrous toluene is added dropwise 5.3 g (0.042 mole) of 3-
chloropropionyl chloride, and the mixture is refluxed at 110-120°C for 4
hours, followed by clarifying the mixture with activated carbon and cooling it
to room temperature. A precipitate of ethyl 10-(3-chloropropionyl)-
phenthiazine-2-carbamate is removed by filtration. The yield is 10.2 g (77.5%
of the theoretical amount), M.P. 169-170°C.
10.2 g of ethyl 10-(3-chloropropionyl)-phenthiazine-2-carbamate ester is
dissolved in 50 ml of toluene, 4.72 g of morpholine is added thereto, and the
mixture is refluxed at 110-120°C for a period of 3 hours. A precipitate of
morpholine hydrochloride is removed by filtration, and the filtrate is washed
with water in order to remove excess morpholine, followed by acidulating with
dilute hydrochloric acid to adjust the pH of the filtrate is adjusted at 3. The
acidic aqueous layer is separated, clarified by treatment with activated carbon
and made alkaline until the pH equals 8-9. This procedure yields the free base
of ethyl 10-(β-morpholylpropionyl)-phenthiazine-2-carbamate, M.P. 156-
157°C.
The free base thus obtained is extracted with toluene, the extract is dried over
magnesium sulphate and to the anhydrous toluene solution is added an
anhydrous ethereal solution of hydrogen chloride until the precipitation of the
target compound is complete. This procedure yields 9.53 g (76.2% of the
theoretical amount) of ethyl 10-(β-morpholylpropionyl)-phenthiazine-2-
carbamate hydrochloride. After recrystallization from dichloroethane, the
target compound melts at 189°C. (decomp.).
brand name
Ethmozine (Shire).
Therapeutic Function
Antiarrhythmic
Properties of MORACIZINE HYDROCHLORIDE
| Melting point: | 189° (dec) |
| form | Solid |
| color | Off-white to light brown |
| EPA Substance Registry System | Moricizine hydrochloride (29560-58-5) |
Safety information for MORACIZINE HYDROCHLORIDE
Computed Descriptors for MORACIZINE HYDROCHLORIDE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1