MonoMethyl auristatin E
- CAS NO.:474645-27-7
- Empirical Formula: C39H67N5O7
- Molecular Weight: 717.98
- MDL number: MFCD22124498
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
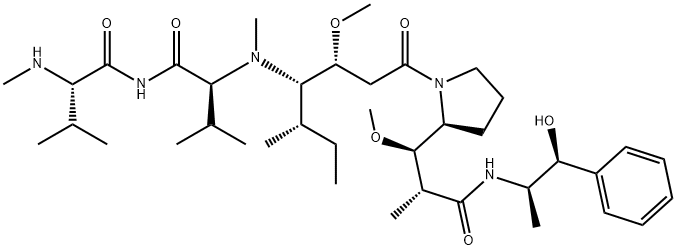
What is MonoMethyl auristatin E?
Description
MMAE is a synthetic antineoplastic agent. Because of its toxicity, it cannot be used as a drug itself; instead, it is linked to a monoclonal antibody (MAB) which directs it to the cancer cells. Monomethyl auristatin E or MMAE is 100-1000 times more potent than doxorubicin. It is a very potent antimitotic agent that inhibits cell division by blocking the polymerization of tubulin.
Chemical properties
Monomethyl auristatin E (MMAE) is a synthetic antineoplastic agent. Because of its toxicity, it cannot be used as a drug itself; instead, it is linked to a monoclonal antibody (MAB) which directs it to the cancer cells. In International Nonproprietary Names for MMAE-MAB-conjugates, the name vedotin refers to MMAE plus its linking structure to the antibody. It is a potent antimitotic drug derived from peptides occurring in marine shell-less mollusc Dolabella auricularia called dolastatins which show potent activity in preclinical studies, both in vitro and in vivo, against a range of lymphomas, leukemia and solid tumors. These drugs show potency of up to 200 times that of vinblastine, another antimitotic drug used for Hodgkin lymphoma as well as other types of cancer.
MMAE is actually desmethyl-auristatin E; that is, the N-terminal amino group has only one methyl substituent instead of two as in auristatin E itself.
The Uses of MonoMethyl auristatin E
Dolastatin 10 is a natural antimitotic and antineoplastic agent that binds to tubulin and inhibits tubulin polymerization. Monomethyl Auristatin E (MMAE) is a synthetic analog of dolastatin 10 that similarly inhibits tubulin polymerization and exhibits potent cytotoxicity. It is commonly conjugated with monoclonal antibodies directed at antigens specific to cancer cells for tumor-directed cytotoxicity. MMAE is typically coupled to the antibody via a protease-cleavable linker, allowing separation of the drug from the antibody following intracellular localization.[Cayman Chemical]
The Uses of MonoMethyl auristatin E
MMAE is an effective and synthetic cytotoxic analog of Dolastatin 10 which is a potent antimitotic polypeptide isolated from a marine animal.
Biological Activity
monomethyl auristatin e(mmae) is a potent antimitotic agent by blocking the polymerisation of tubulin.microtubules play essential role in the function of the cell. microtubules are also reported to be involved in migration, transport and reorganization and have numerous dynamic roles including movement through motor proteins such as dynein and kinesin and the separation and segregation ofchromosomes during cell division.
Biochem/physiol Actions
Monomethyl Auristatin E (MMAE) is a highly potent peptidyl antimitotic agent that blocks the polymerization of tubulin. Monomethyl Auristatin E is a component of a clinically approved antibodydirected conjugates Brentuximab vedotin, Glembatumumab vedotin, Enfortumab vedotin and Polatuzumab vedotin.
in vitro
the cytotoxic effects of the mmae conjugates on h3396 cells were determined using both pulsed and long-term drug exposure assays. it was found that under both exposure conditions, high degrees of immunological specificity were obtained with the val-cit conjugates. cbr96-val-cit-mmae was highly active at <1/100th of the concentration required for antigen saturation [1].
in vivo
in vivo therapy tests were undertaken in athymic mice with subcutaneous l2987 human lung adenocarcinoma xenografts. mmae conjugates were administered at 3 mg mab component/kg/dose. all of the tested mmae conjugates were highly efficacious, leading to long-term regressions of established tumors, whereas the nonbinding control conjugates had no effect on tumor growth. in addition, there were no apparent toxicities associated with conjugate treatment [1].
References
[1] doronina so,toki be,torgov my,mendelsohn ba,cerveny cg,chace df,deblanc rl,gearing rp,bovee td,siegall cb,francisco ja,wahl af,meyer dl,senter pd. development of potent monoclonal antibody auristatin conjugates for cancer therapy. nat biotechnol.2003 jul;21(7):778-84.
[2] jian xu*, priya agarwal, ola saad, et al. clinical pharmacokinetics (pk) of anti-muc16 antibody-drug conjugates (adcs), dmuc5754a, in patients with platinum-resistant ovarian cancer: results from phase i study. this poster was presented at world adc summit 2014.
Properties of MonoMethyl auristatin E
| Melting point: | >90°C (dec.) |
| Boiling point: | 873.5±65.0 °C(Predicted) |
| Density | 1.088±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Slightly, Sonicated) |
| form | Solid |
| pka | 13.66±0.20(Predicted) |
| color | White to Off-White |
| CAS DataBase Reference | 474645-27-7 |
Safety information for MonoMethyl auristatin E
| Signal word | Warning |
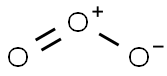
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for MonoMethyl auristatin E
| InChIKey | RJACXSRFJLSJEZ-UIJRFTGLSA-N |
| SMILES | C(NC(=O)[C@H](C(C)C)NC)(=O)[C@H](C(C)C)N([C@@H]([C@@H](C)CC)[C@H](OC)CC(N1CCC[C@H]1[C@H](OC)[C@@H](C)C(N[C@H](C)[C@@H](O)C1=CC=CC=C1)=O)=O)C |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Monomethyl auristatin E 98% (HPLC) CAS 474645-27-7View Details
Monomethyl auristatin E 98% (HPLC) CAS 474645-27-7View Details
474645-27-7 -
 Monomethyl auristatin e 97% CAS 474645-27-7View Details
Monomethyl auristatin e 97% CAS 474645-27-7View Details
474645-27-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1