Ozone
- CAS NO.:10028-15-6
- Empirical Formula: O3
- Molecular Weight: 47.9982
- MDL number: MFCD04113263
- EINECS: 233-069-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
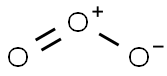
What is Ozone?
Description
Ozone in the upper layers of the atmosphere (stratosphere) is formed by the reaction of O2 with the elemental oxygen formed from the splitting of O2 by UV radiation. The ozone layer in the stratosphere, though containing a relatively low amount of O3 relative to O2, absorbs UV radiation and serves to protect Earth fromthe destructive andmutagenic properties of solarUV.Ozone is more unstable than O2 and thus more reactive. Ground-level ozone (troposphere) is formed largely fromthe reactionof the byproducts of the incomplete combustion of fossil fuels with elemental oxygen present. Common industrial pollutants and car exhaust by-products such asnitrogen oxides, sulfur oxides, carbon oxides, and hydrocarbons are photochemically cleaved and then react with the O2 present. Natural sources of tropospheric ozone come from ozone migration from the stratosphere with average concentration of about 10–20 ppb in nonurban areas. Tropospheric ozone can be very harmful to human health, and people with conditions such as emphysema, asthma, bronchitis, and heart conditions are especially susceptible. Health effects from ozone are due to its high reactivity resulting in reactions with biological macromolecules and subsequent cellular damage. In addition, ozone conversion to diatomic oxygen results in the production of free oxygen radicals, which can also cause damage. Due to the harmful health effects of ozone, theUS Environmental Protection Agency (EPA) among other government entities has established exposure limits. The National Ambient Air Quality Standards limit for ozone is 0.075 ppm, taken as the annual fourth-highest dailymaximum8 h concentration, averaged over 3 years. Ozone exceeds its limit more often than all other regulated air chemicals, and is in excess most frequently in California.
Description
Ozone (O3) plays an extremely important role in the upper atmosphere (stratosphere) and lower atmosphere (troposphere). In the stratosphere (10–50 km above sea level), UV radiation breaks O2?molecules into atomic oxygen (O); O2?and O then recombine to form O3. This first step absorbs almost all UV-C radiation (200–280 nm) from the sun before it passes below ~40 km above sea level; this is the rate-limiting step in O3?formation.
O3?is photolyzed by UV-B radiation (280–320 nm) back to O and O2. This process absorbs a large amount of UV-B radiation, but some still reaches Earth’s surface where it is the primary cause of sunburn. With depleted amounts of O3?in the atmosphere, more UV-B radiation reaches sea level.
A significant amount of Earth’s O3?(~10%) exists in the troposphere (~0–10 km). Here it is not produced by the photolysis of O2?because the required UV-C radiation has been absorbed in the stratosphere. Instead, O3?is produced primarily during the oxidation of CH4?and NO. Anthropogenic generation of these compounds has greatly increased over the past century and led to an increase in tropospheric O3, which is linked to health problems such as asthma.
Chemical properties
Ozone,O3, a colorless gas or dark blue liquid, also known as activated oxygen, is an allotropic form of oxygen formed in nature by lightning in air and during evaporation of water particularly by spray in the sea.It is an unstable blue gas with a distinctive odor. Condenses to a blue black liquid or crystalline solid. Ozone absorbs ultraviolet rays and acts as a natural blanket that protects the earth from harmful short-wave radiation from the sun. Ozone is a powerful oxidizer. It is used as an oxidant in the rubber industry, as a bleaching agent,as a water purifier, and to treat industrial wastes.
Physical properties
Ozone is an allotropic molecular form of oxygen containing three atoms of oxygen (O3).It is a much more powerful oxidizing agent than diatomic oxygen (O2) or monatomic oxygen(O). It is the second most powerful oxidizer of all the elements. Only fluorine is a strongeroxidizer. It is not colorless as is oxygen gas. Rather, ozone is bluish in the gaseous state, butblackish-blue in the liquid and solid states (similar to the color of ink).
Ozone’s boiling point is –112°C, and its freezing point is –192°C.
Origin of Name
From the Greek words oxys (which means sharp or acid) and gen (which means forming); together they stand for “acid-forming.” In the eighteenth century, it was believed that all acids contained oxygen.
Characteristics
Ozone has a very distinctive pungent odor. It exists in our lower atmosphere in very smalltrace amounts. In higher concentrations it is irritating and even poisonous. Ozone is in relativelylow concentrations at sea level. In the upper atmosphere, where it is more concentrated,it absorbs ultraviolet radiation, which protects the Earth and us from excessive exposure toultraviolet radiation.
Electrical discharges in the atmosphere produce small amounts of ozone. You can recognizethe odor when running electrical equipment that gives off sparks. Even toy electric trainscan produce ozone as they spark along the track. Ozone can be produced by passing dry airbetween two electrodes that are connected to alternating electric current with high voltage.Such a system is sometimes used to purify the air in buildings or provide ozone for commercialuses. Ozone is produced during the electrical discharges of lightning during storms. Thisis what makes the air seem so fresh after a thunderstorm or electrical storm. Besides beingproduced by electrical discharges, ozone is produced in the upper atmosphere or stratosphereby ultraviolet (UV) radiation from the sun strikingO2 molecules, breaking them down andreforming them as O3 molecules. The vast majority of ozone is produced in the atmosphereover the tropical latitudes because this area gets most of the radiation from more direct sunlight. Normal wind currents carry the ozone to the polar regions of the Earth where it isthickest.
History
It was once believed that air was a single element, but by the fifteenth century ce, scientistsbegan to question whether it was possibly at least two separate gases. Leonardo da Vinci wasone of the first to suggest the air consisted of at least two gases. He even determined that oneof them would support life and fire.
In 1839 Christian Friedrich Schonbein (1799–1868) discovered a gas with an unusualodor coming from some electrical equipment. He did not know what it was, but because ithad an odd smell, he called it “ozone,” after the Greek word for “I smell.” Although he knewthat it was a chemical substance, he mistakenly associated ozone with the halogens (group 17).Others before Schonbein had smelled the gas but had not recognized its importance. ThomasAndrews (1813–1885) and several other scientists, through different experiments, identifiedozone as a form of oxygen (an allotrope). It was not until 1868 that J. Louis Soret establishedthe formula to be O3.
The Uses of Ozone
Ozone is used as an oxidizing compound, as a disinfectant for air and water, for bleaching waxes and oil, and in organic synthesis. It occurs in the atmosphere at sea level to about 0.05 ppm. It is produced by the action of ultraviolet (UV) radiation on oxygen in air.
The Uses of Ozone
As disinfectant for air and water by virtue of its oxidizing power. For bleaching waxes, textiles, oils. In organic syntheses. Forms ozonides which are sometimes useful oxidizing Compounds.
The Uses of Ozone
Ozone is much more reactive than O2, which makes it a very powerful oxidizing agent.Only fluorine is more reactive. It has many commercial uses. It is a strong oxidizer, particularlyof organic compounds, it is a strong bleaching agent for textiles, oils, and waxes, and it is apowerful germicide. It is also used in the manufacture of paper, steroid hormones, waxes, andcyanide and in the processing of acids.
Ozone produced by electrical discharge is used to purify drinking water and to treatindustrial wastes and sewage. It is also use to deodorize air and kill bacteria by passing dry airthrough special ozone-producing electronic devices.
Definition
A poisonous, blue-colored allotrope of oxygen made by passing oxygen through a silent electric discharge. Ozone is unstable and decomposes to oxygen on warming. It is present in the upper layers of the atmosphere, where it screens the Earth from harmful short-wave ultraviolet radiation. There is concern that the ozone layer is possibly being depleted by the use of fluorocarbons and other compounds produced by industry.
Production Methods
Ozone (triatomic oxygen) is a light blue gas with a characteristic
odor (reminiscent to some individuals of an electrical
discharge such as lightening). Ozone was first
described in 1840 by Christian Friedrich Schonbein
[1799–1868], who produced it from phosphorus and electrolysis
of water. Schonbein also developed a colorimetric assay
involving starch and potassiumiodide-impregnated paper that
was widely used to measure atmospheric ozone concentrations.
Interestingly, Schonbein’s studies were interrupted
when he discovered the acute toxicity of ozone in 1851 and
noted that ozone caused “a really painful affection of the chest,
a sort of asthma, connected with a violent cough”.
Concern of ozone’s toxicity dates back to the mid-twentieth century, when it was recognized as a major air pollutant in
urban areas. Additional concerns arose in the 1980s and 1990s
regarding its depletion in the stratosphere.
Ozone can be found naturally in the troposphere during
electrical storms and in the stratosphere. Background levels
of ozone in nonurban areas average about 10–20 ppb and are
due mainly to intrusion of stratospheric ozone into the lower
atmosphere.
Preparation
Ozone is generated from oxygen by passing an electric spark or silent electrical discharge through dry, and pure oxygen. This electrical discharge may be applied between two glass surfaces between which oxygen is passed. Many types of ozonizers (ozone generating apparatus) are known and commercially available for small-scale production of this gas for various uses. Ozone may be produced by electrolysis of chilled dilute sulfuric acid (e.g. 2.5N H2SO4) or perchloric acid at high current density (higher than that required to produce oxygen alone). A mixture of oxygen and ozone evolve at the anode.
Reactions
Ozone reacts (1) with potassium iodide, to liberate iodine, (2) with colored organic materials, e.g., litmus, indigo, to destroy the color, (3) with mercury, to form a thin skin of mercurous oxide causing the mercury to cling to the containing vessel, (4) with silver film, to form silver peroxide, Ag2O2, black, produced most readily at about 250 C, (5) with tetramethyldiaminodiphenylmethane (CH3)2N·C6H4·CH2·C6H4·N(CH3)2, in alcohol solution with a trace of acetic acid to form violet color (hydrogen peroxide, colorless; chlorine or bromine, blue; nitrogen tetroxide, yellow). In contrast to hydrogen peroxide, ozone does not react with dichromate, permanganate, or titanic salt solutions. Ozone reacts with olefin compounds to form ozonide addition compounds. Ozonides are readily split at the olefinin-ozone position upon warming alone, or upon warming their solutions in glacial acetic acid, with the formation of aldehyde and acid compounds which can be readily identified, thus serving to locate the olefin position in oleic acid, C17H33·COOH, as midway in the chain (CH3(CH2)7CH:CH(CH2)7COOH. Ozone is used (1) as a bleaching agent, e.g., for fatty oils, (2) as a disinfectant for air and H2O, (3) as an oxidizing agent.
General Description
A colorless to bluish gas that condenses to a dark blue liquid, or blue-black crystals. Has a characteristic odor in concentrations less than 2 ppm. Used as a disinfectant for air and water; used for bleaching waxes, textiles and oils, ozonolysis of unsaturated fatty acids to pelargonic and other acids; manufacture of ink; catalyst; water treatment for taste and odor control; mold and bacteria inhibitor in cold storage; bleaching agent.
Reactivity Profile
Ozone is a propellant; ignites upon contact with alcohols, amines, ammonia, beryllium alkyls, boranes, dicyanogen, hydrazines, hydrocarbons, hydrogen, nitroalkanes, powdered metals, silanes, or thiols [Bretherick 1979. p.174]. Aniline in a atmosphere of Ozone produces a white galatinous explosive ozobenzene [Mellor 1:911. 1946-47]. A mixture of ether and Ozone forms aldehyde and acetic acid and a heavy liquid, ethyl peroxide, an explosive [Mellor 1:911. 1946-47]. Severe explosions occur attempting to form tribromic octaoxide from bromine and Ozone [Mellor 2, Supp. 1:748. 1956]. Mixtures of Ozone and dinitrogen pentaoxide are flammable or explosive [Mellor 8, Supp. 2:276. 1967]. Ozone and ethylene react explosively [Berichte 38:3837]. Nitrogen dioxide and Ozone react with the evolution of light, and often explode [J. Chem. Phys. 18:366 1920]. Contact of very cold liquefied gas with water may result in vigorous or violent boiling of the product and extremely rapid vaporization due to the large temperature differences involved. If the water is hot, there is the possibility that a liquid "superheat" explosion may occur. Pressures may build to dangerous levels if liquid gas contacts water in a closed container, [Handling Chemicals Safely 1980].
Hazard
High concentrations of ozone are a fire and explosion hazard when in contact with anyorganic substance that can be oxidized.
In moderately high concentrations ozone is very toxic when inhaled, and in lesser concentrations,it is irritating to the nose and eyes. Ozone in the lower atmosphere contributes to airpollution and smog. It can cause damage to rubber, plastics, and paints. These low concentrationscan cause headaches, burning eyes, and respiratory irritation. It is particular harmful toasthmatics and the elderly with respiratory problems.
Health Hazard
Ozone is a highly toxic gas that is extremely irritating to the eyes, mucous membranes, and respiratory tract. The characteristic odor of ozone can be detected below the permissible exposure limit, and this compound is therefore regarded to have adequate warning properties. However, at higher concentrations the ability to smell ozone may decrease. Inhalation of 1 ppm ozone may cause headaches and irritation of the upper and lower respiratory tract. The first symptoms of exposure include irritation of the eyes, dryness of throat, and coughing; these symptoms disappear after exposure ceases. Exposure at higher levels may lead to lacrimation, vomiting, upset stomach, labored breathing, lowering of pulse rate and blood pressure, lung congestion, tightness in the chest, and pulmonary edema, which can be fatal. Exposure to 100 ppm of ozone for 1 hour can be lethal to humans. Animal studies indicate that chronic exposure to ozone may result in pulmonary damage, leading to chronic lung impairment. Continual daily exposure to ozone can cause premature aging.
Fire Hazard
Severe explosion hazard when shocked, exposed to heat or flame, or by chemical reaction with organic substances, especially reducing agents. Ozone is a powerful oxidizing agent. Incompatible with alkenes; aromatic compounds; benzene, rubber; bromine; dicyanogen; diethyl ether; dinitrogen tetroxide; hydrogen bromide; 4-hydroxy-4-methyl-1,6-heptadiene; nitrogen trichloride; stibine; tetrafluorohydrazine. Avoid contact with organic materials.
Flammability and Explosibility
Ozone by itself is not flammable. Liquid ozone and concentrated solutions are extremely hazardous and can explode on warming or when shocked.
Agricultural Uses
Ozone (O3) which is triatomic oxygen and measured in Dobson units, is a blue gas with pungent odor. Ozone is made by subjecting oxygen to a high-voltage electric discharge. It is used for killing germs, bleaching, removing unpleasant odors from food and sterilizing water. It is a powerful oxidizing agent and decomposes rapidly above 373°K.
The upper atmosphere contains a layer of ozone, formed when ultraviolet radiation acts on oxygen. It protects the earth from the sun's ultraviolet rays. In recent years there has been significant reduction in the amount of atmospheric ozone. This is due to the discharge of chlorofluorocarbons (CFC) into the atmosphere (both the troposphere and the stratosphere),which are widely used in refrigeration, insulating foam, solvents, aerosol propellants, and chlorine and bromine gases. These remain in the atmosphere for long periods and destroy the ozone layer.
Scientists predict that as the ozone shield thins and allows more ultraviolet radiation to reach the earth, there could be an increased incidence of skin cancer and eye disease among humans, and could cause damage to marine life, crops and forests.
The Montreal Protocol, ratified by 183 countries (by 2002), called for freezing the use of chlorofluorocarbons at the 1986 level, and then rolling back the production in a phased manner. Developed countries have been responsible for the overwhelming contribution toward use of ozone depleting chemicals. With stronger political will, many countries have phased out use of most of the CFCs, halons, methyl bromide and other substances. Developing countries are committed to reducing their CFC production and consumption by 85% in 2007. Regular reviews by United Nations Environmental Program (UNEP) and other world bodies consider that implementation of the Montreal Protocol's provisions are on the right track. Data emerging out of such reviews suggests that atmospheric concentrations of CFCs have declined paving the way for a possible corresponding decrease in global warming. But on the other hand, use of other ozone-depleting substances such as HFC ( hydrofluorocarbons ) and HCFC (hydrochlorofluorocarbons) have been on the rise, causing concern on the future of the ozone layer.
More recent evidence reveals that the Antarctic ozone hole has increased in size and measures 10.6 million square miles. And although some scientists believe that this rate is not as rapid as during the 1980s and that a future Arctic polar ozone hole seems unlikely, many experts consider this as an issue that demands serious attention.
Safety Profile
A human poison by inhalation. Human systemic effects by inhalation: visual field changes, lachrymation, headache, decreased pulse rate with fall in blood pressure, dermatitis, cough, dyspnea, respiratory stimulation and other pulmonary changes. Experimental teratogenic and reproductive effects. Human mutation data reported. A skin, eye, upper respiratory system, and mucous membrane irritant. Questionable carcinogen with experimental neoplas tigenic and tumorigenic data. Can be a safe water dtsinfectant in low concentration. Concentration of 0.01 5 pprn of ozone in air produces a barely detectable odor. Concentrations of 1 ppm produce a dtsagreeable sulfur like odor and may cause headache and irritation of eyes and the upper respiratory tract; symptoms dtsappear after leaving the exposure. A powerful oxidning agent. Dangerous chemical reaction with acetylene, alkenes, alkylmetals (e.g., Imethylzinc, Iethylzinc), antimony, aromatic compounds (e.g., benzene, aniline), benzene + oxygen + rubber, bromine, charcoal + potassium iodide, citronelk acid, combustible gases (e.g., carbon monoxide, ethylene, nitrogen oxide, ammonia, phosphme), (dtallyl methyl carbinol + acetic acid), trans-2,3-dichloro-2butene, dicyanogen, dlenes + oxygen, dtethyl ether, 1,l -Ifluoroethylene, N205, ethylene + formyl fluoride, fluoroethylene, liquid hydrogen, hydrogen + oxygen difluoride, hydrogen bromide, hydrogen iodide, 4-hydroxy-4-methyl-1 ,6-heptadtene, 2,3-hydroxy-2,2,4-trimethyl-3-pentenoic acid lactone, isopropylidene compounds, nitrogen, NOZ, NOx nitrogen trichloride, nitrogen triiodide, nitroglycerin, organic liquids, organic matter, oxygen + rubber powder, oxygen fluorides (e.g., dioxygen difluoride, dioxygen trifluoride), shca gel, stibine, tetrafluorohydrazine, tetramethylammonium hydroxide, trifluoroethylene, unsaturated acetals. A severe explosion hazard in liquid form when shocked, exposed to heat or flame, or in concentrated form by chemical reaction with powerful reducing agents. Incompatible with rubber; dinltrogen tetraoxide. See also OZONIDES and PEROXIDES.
Potential Exposure
Ozone is found naturally in the atmosphere as a result of the action of solar radiation and electrical storms. It is also formed around electrical sources, such as X-ray or ultraviolet generators, electric arcs; mercury vapor lamps; linear accelerators; and electrical discharges. Ozone is used as an oxidizing agent in the organic chemical industry (e.g., production of azelaic acid); as a disinfectant for air, mold and bacteria inhibitor for food in cold storage rooms, and for water (e.g., public water supplies; swimming pools; and sewage treatment); for bleaching textiles; waxes, flour, mineral oils, and their derivatives; paper pulp; starch, and sugar; for aging liquor and wood; for processing certain perfumes; vanillin, and camphor; in treating industrial wastes; in the rapid drying of varnishes and printing inks; and in the deodorizing of feathers.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. Administer 100% O2. Medical observation isrecommended for 24-48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
Carcinogenicity
Ozone has been positive as a
genotoxic substance in certain assay systems, but the results
are inconsistent. For example, in vitro
assays have noted that ozone can induce bacterial mutations,
plasmid DNA strand breakage, chromatid and chromosome
aberrations in lymphocytes, and a doubling of the frequency
of preneoplastic variants compared with control cultures.
However, in vivo assays of similar end points produced mixed
results. For example, alveolar macrophages
from rats exposed to 270–800 ppb ozone developed chromatid
damage, but no chromosomal changes. In human
subjects exposed to 500 ppb ozone (6–10 h), a slight increase
in sister chromatid exchange persisted for ≤6 weeks.
In contrast, no significant changes in chromosome or chromatid
breaks were observed in lymphocytes of subjects
exposed for 4 h to 400 ppb. Cultured human epidermal cells exposed to 500 ppb ozone for 10 min showed no evidence
of DNA strand breakage.
Other investigators have suggested that chronic ozone
exposure may facilitate the development of benign pulmonary
tumors (adenomas) in mice and other hyperplastic
nodules in the lungs of nonhuman primates.
As is true of hyperoxia, ozone exposure may enhance or
retard lung tumorigenesis by other agents in rodents, depending
on the exposure protocol.
Other investigators have suggested that in vitro assays
indicate ozone may exert indirect genotoxic effects. Ozone
has been purported to affect the integrity of immune system
defenses against tumor development and progression
(1073, 1074). In addition, arylamines found in tobacco
smoke (e.g., naphthylamine and toluidine isomers) can be
chemically altered by brief exposures (1 h) to 100–400 ppb
ozone. The unidentified stable products of this reaction cause
single-strand DNA breaks in cultured human lung cells
equivalent to that produced by 100 rad of irradiation.
However, an in vivo cocarcinogenicity study failed to find
similar effects.
Environmental Fate
Ozone formed from anthropogenic sources such as from car vehicle emissions in the troposphere can travel long distances. Ozone formation and scavenging by other chemicals such as NO is in constant daily flux. There are times when solar radiation is high, such as on hot days or during rush hour, which produces elevated ozone levels and times such as during the evening when the rate of ozone scavenging exceeds ozone production, resulting in less ozone in the atmosphere. Ozone concentrations in the eastern United States are often more than 80 ppb in the warm spring and summer months, though ozone levels in the western United States are lower.
storage
Work with ozone should be conducted in a fume hood to prevent exposure by inhalation. Ozone is usually produced in the laboratory with a ozone generator, and care should be taken to ensure adequate ventilation in the area where the ozone generation equipment is located. Because of the possibility of the generation of explosive ozonides, ozonolysis reactions should always be conducted in a fume hood behind a safety shield.
Shipping
UN1955 Compressed gas, toxic, n.o.s, Inhalation Hazard Zone A, Hazard Class: 2.3; Labels: 2.3-Poisonousgas, 5.1-Oxidizer, Technical name required, Inhalation Hazard Zone A. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Toxicity evaluation
The biochemical mechanism of ozone-induced lung injury is due to the reaction of the highly reactive O3 with biological macromolecules such as protein, lipids, nucleic acids, and carbohydrates. The resulting formation of reactive free-radical intermediates from the oxidization of thiol-containing amino acids forms disulfide bonds and methionine sulfoxide. Polyunsaturated fatty acids in cell membrane lipid bilayers are a major target and react with the ozone to induce lipid peroxidation to affect membrane fluidity and induce cellular damage. Nucleic acids can also be affected by the oxidative potential of ozone. The primary site of injury is the lung, and the injury is characterized by pulmonary congestion, edema, and hemorrhage. The area of the lung that is particularly sensitive to ozone is the junction of the bronchioles and the alveoli. Effects of ozone on lung function vary greatly between individuals. Antioxidants present in the respiratory lining protect against oxidative injury.
Incompatibilities
A powerful oxidizer. A severe explosion hazard when exposed to shock or heat, especially solid or liquid form. Spontaneously decomposes to oxygen under ordinary conditions; heating increases oxygen production. Reacts with all reducing agents; combustibles, organic, and inorganic oxidizable materials; and can form products that are highly explosive. Incompatible with alkenes, aniline, benzene, bromine, ether, ethylene, and hydrogen bromide; nitric oxide; stibine. Attacks metals except gold and platinum.
Waste Disposal
Vent to atmosphere. Use a licensed professional waste disposal service to dispose of this material. All federal, state, and local environmental regulations must be observed. Return refillable compressed gas cylinders to supplier.
Properties of Ozone
| Melting point: | 193℃ |
| Boiling point: | -110℃ |
| Density | 1.46 g/cm3 |
| vapor pressure | 55kPa at -12℃ |
| solubility | slightly soluble in H2O |
| form | blue gas |
| appearance | Colorless to pale blue gas |
| color | Blue or violet-black solid or unstable colorless gas or dark-blue liquid |
| Odor | Pungent odor, detectable at 0.01 to 0.04 ppm; sharp disagreeable odor at 1 ppm |
| Odor Threshold | 0.0032ppm |
| Water Solubility | 570mg/L at 20℃ |
| Exposure limits | TLV-TWA 0.1 ppm (~0.2 mg/m3) (ACGIH, NIOSH, and MSHA), 0.2 ppm (~0.4 mg/m3) (OSHA); IDHL 10 ppm (NIOSH). |
| Stability: | Unstable - may decompose spontaneously and violently to oxygen. Mixtures containing a moderate partial pressure of ozone, and pure ozone at even low pressures are both potentially explosive. May react very violently with combustible materials and reducing agents, such as organics. Even small quantities of organic material, such as traces of g |
| EPA Substance Registry System | Ozone (10028-15-6) |
Safety information for Ozone
Computed Descriptors for Ozone
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran






You may like
-
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 2-Hexyn-1-ol 98+View Details
2-Hexyn-1-ol 98+View Details
764-60-3 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
