Molybdenum
- CAS NO.:7439-98-7
- Empirical Formula: Mo
- Molecular Weight: 95.94
- MDL number: MFCD00003465
- EINECS: 231-107-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
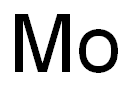
What is Molybdenum?
Description
Molybdenum is an essential trace mineral associated with several enzyme systems required for the normal body functions. Mine workers have developed symptoms of molybdenosis. The significantly expanding or softening property of the material makes it useful in applications that involve intense heat, including the manufacture of aircraft parts, electrical contacts, industrial motors, and filaments.
Chemical properties
Molybdenum is a silvery-white metal or dark gray or black powder with a metallic luster.
Physical properties
Molybdenum is in the middle of the triad elements of group 6. These three metals (fromperiods 4, 5, and 6) are chromium, molybdenum, and tungsten, which, in their pure states,are relatively hard, but not as hard as iron. They are silvery-white as pure metals, and they havesimilar oxidation states. Their electronegativity is also similar—Cr = 1.6, Mo = 1.8, and W =1.7—which is related to their reactivity with nonmetals.
Molybdenum is malleable and ductile, but because of its relatively high melting point, it isusually formed into shapes by using powder metallurgy and sintering techniques.
Molybdenum’s melting point is 2,617°C, boiling point = 4,612°C, and its density is 10.22g/cm3.
Isotopes
There are 36 isotopes of molybdenum, ranging in atomic weights from Mo-83to Mo-115. Of the seven isotopes considered stable, one (Mo-100) is radioactive and isconsidered stable because it has such a long half-life (0.95×10+19 years). The proportionsof the seven stable isotopes contributing to molybdenum’s natural existence onEarth are as follows: Mo-92 = 14.84%, Mo-94 = 9.25%, Mo-95 = 15.92%, Mo-96 =16.68%, Mo-97 = 9.55%, Mo-98 = 24.13%, and Mo-100 = 9.63%.
Origin of Name
Molybdenum is derived from the Greek word molybdos, meaning lead. At one time, the mineral molybdaena (later called molybdenite) was believed to be a variety of lead ore.
Occurrence
Molybdenum is the 54th most abundant element on Earth. It is relatively rare and is foundin just 126 ppm in the Earth’s crust. Its major ore is molybdenite (MoS2), which is mined inColorado in the United States and is found too in Canada, Chile, China, England, Norway,Sweden, Mexico, and Australia. Moldybdenum is also found in two less important ores: wulfenite(PbMoO4) and powellite ([Ca(MoW)O4]. These ores are usually found in the same sitesalong with tin and tungsten ores.
Molybdenite ore is very similar to graphite, and they have been mistaken for each otherin the past.
Characteristics
Given that molybdenum is located between chromium and tungsten in group 6, it chemicallyresembles a cross between these two partner elements. The three related elements donot occur as free elements in nature, but rather are found in minerals and ores. Their metal(elemental) radius size increases from chromium = 44 to molybdenum = 59 to tungsten = 60,which is related to their electronegativity and results in their using electrons in shells inside theouter shell during metallic bonding. This is a major characteristic of the transition of elementsfrom metals to nonmetals.
Molybdenum oxidizes at high temperatures but not at room temperatures. It is insolublein acids and hydroxides at room temperatures. At room temperatures, all three metals(chromium, molybdenum, and tungsten) resist atmospheric corrosion, which is one reasonchromium is used to plate other metals. They also resist attacks from acids and strong alkalis,with the exception of chromium, which, unless in very pure form, will dissolve in hydrochloricacid (HCl).
History
Peter Jacob Hjelm (1746–1813) is given credit for discovering molybdenum in 1781despite the fact that his paper was not published until 1890. He followed the advice of CarlWilhelm Scheele (1742–1786), who isolated and identified molybdenum, but incorrectlythought it was an element related to lead.
Although some reference works do give Scheele credit, most do not credit him for thediscovery of either molybdenum or the other elements he “discovered,” such as oxygen andmanganese.
Scheele did not receive credit for discovering oxygen two years before Joseph Priestley(1733–1804) announced his discovery and was given the credit. Scheele’s publisher wasnegligent in getting his work published in time. (There is a lesson in this story for all youngscientists—keep completed and accurate records of all your lab work and observations, andwhen you are sure of your experimental results, make sure to publish.)
The name “molybdenum” is derived from the Greek word for lead, molybdos, which standsfor any black minerals that historically could be used for writing. This also explains why theGreek word plumbago or “black lead” was used for graphite.
The Uses of Molybdenum
In the form of ferromolybdenum for manufg special steels for tools, boiler plate, rifle barrels, propeller shafts; electrical contacts, spark plugs, x-ray tubes, filaments, screens and grids for radio tubes; in the production of tungsten; glass-to-metal seals; nonferrous alloys; in colloidal form as lubricant additive.
The Uses of Molybdenum
Molybdenum is largely used in steel industry.Its compounds are widely used incoloring agents, solid lubricants and ascatalysts. Molybdenum is an essential traceelement and a component of xanthine oxidase.This enzyme catalyzes the formationof urate. Molybdenum cofactor (Moco)-deficiencyis a lethal autosomal recessive disease.Moco-deficiency in humans can causeneurological damage, seizures and variousbrain dysmorphisms.
The sources of molybdenum include beans,dark green leafy vegetables, grains and hardtap water. Deficiencies of molybdenum arealmost unknown in human.
The Uses of Molybdenum
The high melting point of molybdenum is the major determinant of how it is used. Its chiefuse is as an alloy in the manufacture of engines of automobiles. “Moly-steel” contains up to8% molybdenum and can withstand high pressures and the relatively rapid changes of enginetemperatures (e.g. cold engine to hot and back again without the metal warping and with theability to withstand excessive expansion and contraction).
Its high melting point also makes it useful for metal electrodes in glassmaking furnaces.Molybdenum’s high resistance to electricity makes it useful in high-temperature filament wiresand in the construction of parts for missiles, spacecrafts, and nuclear power generators.
Molybdenum is also used as a catalyst in petroleum refining, as a pigment for paints andprinter’s ink, and as a high-temperature lubricant (molybdenum disulphide-MoS2) for use byspacecraft and high-performance automobiles.
In hospitals, radioisotope Mo-99, which decays into technetium-99, is given internally tocancer patients as a “radioactive cocktail.” Radioactive Tc-99 is absorbed by tissues of cancerpatients, and then x-ray-like radiation is used to produce pictures of the body’s internal organs.
Background
Molybdenum is a chemical element with symbol Mo and atomic number 42. As it is not a naturally occuring free metal on Earth, molybdenum is found only in various oxidation states in minerals. Molybdenum is commonly used in metallurgy and other chemical applications however it has essential biological roles in organisms and microorganisms. It is an essential trace dietary element and acts as a critical cofactor in several molybdenum-dependent enzymes that are involved in important cellular reactions and pathways, including xanthine oxidoreductase. When complexed with Technetium Tc 99m, molybdenum is used as a radiopharmaceutical agent in diagonistic procedures.
Definition
Metallic element of atomicnumber 42, group VIB of the periodic table, aw95.94, valences = 2, 3, 4, 5, 6. Seven stable isotopes.
Definition
A transition element that occurs naturally in molybdenite (MoS2) and wulfenite (PbMoO4). It is used in alloy steels, lamp bulbs, and catalysts. The compound ammonium molybdate, dissolved in nitric acid, is used as a test for phosphates(V). Molybdenum sulfide (MoS2) is used in lubricants to enhance viscosity. Symbol: Mo; m.p. 2620°C; b.p. 4610°C; r.d. 10.22 (20°C); p.n. 42; r.a.m. 95.94.
Production Methods
Molybdenum (Mo) is a dark gray or a black powder with a metallic luster and a chemical element of the second transition series. The name is derived from the Greek molybdos, meaning“lead.”In1778,CarlScheeleofSwedenrecognized molybdenite as a distinct ore of a new element, and in 1781 Hjelm prepared an impure form of the metal. The ?rst molybdenum mine, Knaben Gruver mine in southern Norway, was opened in 1885 and remained open until 1973. Today, the principal molybdenum mines are found in the United States, Chile, and China. Mined ore is crushed in ball or rod mills, and metallic minerals are separated from gangue by ?otation. The pure metal is prepared by the reduction of puri?ed molybdic trioxide or ammonium molybdate (AM) with hydrogen. When Mo is a by-product of copper mining, a concentrate ofcopperandmolybdenumis?rstproduced,andthetwoores are later separated by differential ?otation. MoS2 concentrates contain more than 85% MoS2 and roasted MoO3 typically contains a minimum of 57% Mo and less than 0.1% S.
General Description
Molybdenum, a chemical element, is a hard, high-melting (refractory) high-density dark gray metal or black powder. Insoluble in water. Used to make structural alloys; used as a catalyst. Molybdenum dust and fumes can irritate the eyes and respiratory tract.
Reactivity Profile
Molybdenum is a reducing agent. In dust or powder form, Molybdenum may present a fire or explosion hazard under favoring conditions of particle size, dispersion and ignition. Bulk Molybdenum (rod, coil, sheet, etc.) is less reactive than dust or powder. Insoluble in hydrochloric acid or hydrofluoric acid solutions and in ammonia and sodium hydroxide solutions. Insoluble in dilute sulfuric acid solutions but soluble in concentrated sulfuric acid. Soluble in concentrated nitric acid. Incompatible with strong oxidizing agents such as bromine trifluoride, bromine pentafluoride, chlorine trifluoride, potassium perchlorate, nitryl fluoride, fluorine, iodine pentafluoride, sodium peroxide, lead dioxide.
Hazard
Flammable in form of dust or powder.Lower respiratory tract irritant. Questionable car-cinogen.
Hazard
The powder and dust forms of molybdenum are flammable. The fumes from some of thecompounds should not be inhaled or ingested.
Health Hazard
The toxicity of molybdenum in humans isconsidered to be low. Gout-like symptoms,hyperuricaemia and pneumoconiosis havebeen associated with excessive exposures.Selden et al. (2005) have reported a case ofhyperuricaemia and gouty arthritis in a youngman from occupational exposure to molybdenum.Momcilovic (1999) has cited a caseof acute clinical poisoning resulting from thedietary intake of molybdenum supplement ina male patient. A cumulative dose of 13.5 mgMo over a period of 18 days was attributed tocause acute psychosis with visual and auditoryhallucination and a series of petit malseizures. The symptoms decreased severalhours after the start of chelation therapy withcalcium ethylene diamine tetraacetic acid.However, 1 year after such Mo poisoning thepatient was diagnosed toxic encephalopathywith learning disability, depression, and posttraumaticdisorder. Spectral emission computertomography demonstrated evidence offrontal cortical damage of the brain.
Vyskocil and Viau (1999) assessed molybdenumtoxicity in humans and calculated the‘tolerable daily intake’ (TDI), ‘no observedadverse effect level’ (NOAEL) and the ‘lowestobserved adverse effect level’ (LOAEL)for molybdenum intake. The authors havecalculated a TDI of 0.009 mg Mo/kg/dayand a NOAEL and LOAEL of 0.9 and1.6 mg Mo/kg/day respectively. Their toxicologicalrisk analysis was based on animaldata.
Molybdenum toxicity has been found tobe associated with copper deficiency in thebody.
Thus, any copper deficiency arising eitherfrom inadequate dietary intake or from somedysfunction in copper metabolism that woulddeplete its level may contribute to greaterrisk of molybdenum toxicity in humans.
Flammability and Explosibility
Not classified
Agricultural Uses
Molybdenum (Mo) is a plant micronutrient, absorbed by
the plant roots only in the form of molybdate ion MoO42-
Molybdenum, is a vital component of the enzyme,
nitrate reductase (a soluble molybdoflavoprotein) in
Molybdenum is present in the chloroplasts of leaves.
It is also a structural component of nitrogenase which
plays an active role in nitrogen fixation by Rhiwbium,
Azotobucter and some algae and actinomycetes.
Molybdenum is also involved in the absorption and
translocation of iron in plants.
Molybdenum is present in soils in extremely small
quantities (about 2 ppm or less) which is adequate for plants. Its availability increases as the soil pH increases
when the conversion of molybdenum oxide to molybdate
is favored.
The presence of aluminum, iron and titanium in soil
increases the absorption of molybdenum. Nitrate
nitrogen encourages molybdenum uptake, while
ammoniacal nitrogen reduces it. A heavy application of
phosphatic fertilizers increases the uptake of
molybdenum while that of sulphates has the reverse
effect. The addition of lime increases the availability of
molybdenum. A dose of concentrated soluble manganese
and/or copper reduces molybdate absorption by plants.
When the molybdenum content of plants is less than
0.2 ppm, molybdenum deficiency occurs. The
deficiency is determined by the ammonium oxalate
extraction procedure. Acidic soils, fibrous peat soils and
acidic sandy soils are generally molybdenum deficient.
Molybdenum concentration is high at the soil surface, and
decreases with depth; the deficiency is severe under dry
soil conditions, probably owing to reduced diffusion or
mass flow.
Molybdenum deficiency resembles nitrogen
deficiency in legumes, because of the role molybdenum
plays in nitrogen fixation. The deficiency causes stunting
and yellowing of plants. In legume crops, the deficiency
manifests itself by marginal scorching, curling and
crinkling of leaves, the first symptom being
an interveinal chlorosis followed by the plant turning pale
yellow and becoming stunted.
Mo deficiency, manifested in cauliflower, is known as
whiptail and that in cashew, the yellow leaf spot. The deficiency is most common in acidic
sandy soils because of the leaching losses. Molybdenum
availability is low in soils with high metal oxides.
Cauliflower, Brussels sprouts, broccoli and citrus fruits
are sensitive to low molybdate levels. Cotton,
leafy vegetables, corn, tomato and sweet potato are
moderately sensitive to lower molybdate levels.
Molybdenum deficiency also causes nitrate
accumulation, thereby lowering the activity of ascorbic
acid oxidase, known for activating enzymes, namely,
nitrate reductase and xanthine oxidase.
Molybdenum deficiency can easily be corrected by
adding 40 to 400g Mo/ha to the soil. It can also be
corrected by a foliar spray of sodium molybdate or
molybdic acid, or by coating seeds with sodium molybdate before planting. Even a 0.03% foliar spray on
cashews can correct the deficiency. Soil is limed to
increase plant uptake of molybdenum.
A trace of molybdenum as impurity in
superphosphate is often adequate for plant growth. A
material carrying at least 38% molybdenum, such as
sodium molybdate, is employed to coat the seed, about 17
g/ha of which is used for legumes on molybdenumdeficient
soils. Such seed treatment is the most practical
way for augmenting the molybdenum content of the soil.
Ammonium molybdate is recommended for potato at
the rate of about 1 kg/ha for soil application, 0.5 kg in
lo00 liters of water for sprays and 200 g for soaking seed
tubers. A disease, called pencil point, in coconut can be
cured with a 0.25 g/l of ammonium molybdate as a
component of the fertilizer mixture.
Many fertilizers contain molybdenum. The common
ones are ammonium molybdate (54%), sodium
molybdate (39%) and molybdenum trioxide (66%) - the figures in brackets indicating the molybdenum
percentage. Among these, only ammonium molybdate is
recognized under the Fertilizer Control Order (FCO) in
some countries (like India), although sodium molybdate
is the most commonly recommended molybdenum
carrier. Molybdenum is mixed with NPK fertilizer and
then applied. It was demonstrated in Australia that
soaking seeds in sodium molybdate solution before
sowing was as effective as applying molybdenum in the
fertilizer. In the United States, the seeds are often coated
with sodium molybdate.
Molybdenum toxicity is associated with vein
clearing, necrosis, and golden yellow dissolution in the
middle of the lamina in plants because of the formation of
molybdeocatechol complex. Excess of molybdenum is
toxic to animals feeding on forage rich in molybdenum.
Molybdenosis, is a cattle disease, caused by a copper and
molybdenum imbalance in the diet, with the molybdenum
content exceeding 5 ppm. Molybdenum toxicity
provokes stunted growth and bone deformation in
animals. This disease is also known as 'teart' in England
and 'peat scow' in New Zealand. Injecting copper or
adding copper fertilizer to the grazing areas can correct
the imbalance.
As already mentioned, soils generally contain very
low quantities of molybdenum. In most Indian soils
(other than acidic soils), for instance, the total available
molybdenum ranges from traces to a fraction of 1 ppm,
and even with this minute quantity the soil exhibits no
molybdenum deficiency.
Industrial uses
Molybdenum (Mo) is a silvery-white metal, occurring chiefly in the mineral molybdenite but also obtained as a by-product from copper ores. It is ductile, softer than tungsten, and is readily worked or drawn into very fine wire.
Its major use is in alloy steels, for example, as tool steels ( 10% molybdenum), stainless steel, and armor plate. Up to 3% molybdenum is added to cast iron to increase strength. Up to 30% molybdenum may be added to iron-, cobalt-, and nickel-base alloys designed for severe heat- and corrosion-resistant applications. It may be used in filaments for lightbulbs, and it has many applications in electronic circuitry.
Molybdenum forms mirrors and films on glass when it is produced by gas-phase reduction or decomposition of volatile molybdenum compounds in glass tubes. Molybdenum trioxide (MoO3) dissolves in glass, allowing strong binding of molten glass with preoxidized metal surfaces. Annealing is very effective, with little or no difference in thermal expansion at the metal glass interface. Molybdenum found early use in filaments for electric lightbulbs and later in the construction of electronic devices (for example, in vacuum tubes, contacts, electrodes, and transistors).
Safety Profile
Poison by intratracheal route. Mutation data reported. An experimental teratogen. Experimental reproductive effects. Flammable or explosive in the form of dust when exposed to heat or flame. Violent reaction with oxidants (e.g., bromine trifluoride, bromine pentafluoride. chlorine trifluoride, potassium perchlorate, nitryl fluoride, fluorine, iodine pentafluoride, sodium peroxide, lead dioxide). When heated to decomposition it emits toxic fumes of Mo.
Potential Exposure
Most of the molybdenum produced is used in alloys: steel, stainless steel; tool steel; case iron; steel mill rolls; manganese, nickel, chromium, and tungsten. The metal is used in electronic parts (contacts, spark plugs, X-ray tubes, filaments, screens, and grids for radios); induction heating elements; electrodes for glass melting; and metal spraying applications. Molybdenum compounds are utilized as lubricants; as pigments for printing inks; lacquers, paints, for coloring rubber animal fibers, leather, and as a mordant; as catalysts for hydrogenation cracking; alkylation, and reforming in the petroleum industry; in Fischer Tropsch synthesis; in ammonia production; and in various oxidation-reduction and organic cracking reactions; as a coating for quartz glass; in vitreous enamels to increase adherence to steel; in fertilizers, particularly for legumes; in electroplating to form protective coatings; and in the production of tungsten. Hazardous exposures may occur during high-temperature treatment in the fabrication and production of molybdenum products, spraying applications; or through loss of catalyst. MoO3 sublimes above 800℃.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
Guinea pigs exposed to molybdenum trioxide dust at a concentration of 200mg molybdenum/ m3 for 1 hour daily for 5 days developed nasal irritation, diarrhea, weight loss, and incoordination. 3 In 2-year inhalation studies at concentrations of up to 100mg/m3 molybdenum trioxide there was no evidence of carcinogenic activity in female rats, but there was equivocal evidence in males based on a marginally significant positive trend of alveolar/bronchiolar adenoma or carcinoma (combined). There was some evidence of carcinogenic activity in mice based on increased incidences of alveolar/bronchiolar adenoma and carcinomas (combined).4 Other exposure-related effects in exposed animals included alveolar inflammation, squamous metaplasia of the epiglottis and hyaline degeneration of the respiratory and olfactory epithelium. Molybdenum trioxide was not mutagenic in bacterial assays, nor did it induce sister chromatid exchanges or chromosomal aberrations in vitro.
Environmental Fate
Molybdenum is a silvery-white transition metal in Group 6
between chromium and tungsten on the periodic table. It is
mined as a principal ore, and is also recovered as a byproduct of
copper and tungsten mining. Molybdenum does not react with
oxygen or water at room temperature, and the bulk oxidation
occurs at temperatures >790℃, resulting in molybdenum
trioxide, MoO3. Other common molybdenum compounds
commonly encountered include molybdenum trioxide,
sodium molybdate, Na2MoO4.2H2O, and ammonium di- and
heptamolybdate, (NH4)2Mo2O7, and (NH4)6Mo7O24.4H2O.
In aqueous solution, molybdenum is present as the simple
molybdate [MoO4]2- ion which is similar to sulfate or a polymeric
polymolybdate ion. The lower oxidation state is found in
the commonest ore of molybdenum the disulfide, MoS2. The
majority of atmospheric molybdenum emissions are anthropomorphic
sources such as its use in alloys, flame retardants,
smoke oppressors, catalysts, lubricants, and corrosion inhibitors,
and also by mining activities, the application of biosolids
and fertilizers, and atmospheric deposition from smelters. Coal
combustion is the largest atmospheric source of molybdenum.
In water, molybdenum exists primarily as the molybdate ion or
various polymeric compounds depending upon the pH. In
soils, molybdate is sorbed primarily to high-calcium, highchloride
soils with retention lesser in low-sulfate soils.
The primary pathway for molybdenum exposure is ingestion
by water or food. Molybdenum is found in leafy vegetables,
legumes, meat, and many grains. Molybdenum does not
appear to be absorbed dermally. Molybdenum dusts and
fumes, which can be generated by mining or metalworking,
may be inhaled. The concentrations of molybdenum in the
ambient air are normally low compared with other trace
elements; in urban areas, molybdenum ranged from 0.01 to
0.03 mg m-3, and in nonurban areas it varied between 0.001
and 0.0032 mg m-3. Fruits, root vegetables, and muscle meat
are poor sources of molybdenum; however, high concentrations
have been found in shellfish and fish, which contain
about 1 mg kg-1, and plants, which contain 0.03–5 mg kg-1.
Molybdenum levels in drinking water range from 0 to 68 μg l-1,
but usually do not exceed 10 μg l-1. Bioconcentration in most
fish appear to be exposure concentration dependent because it
has been demonstrated that at low environmental concentrations,
molybdenum is concentrated, whereas at high environmental
concentrations it is not concentrated. Molybdenum
levels are elevated in terrestrial flora near anthropomorphic
sources such as mining, fossil fuel plants, and industrial waste
sites. In these areas, the concentrations of molybdenum in
fish, wildlife, and invertebrates were low when compared with
those in terrestrial plants; therefore, there may be some bioconcentration
occurring in the flora. In addition, aquatic flora
and fauna seem to be comparatively resistant to molybdenum
salts and this evidence further indicates a lack of bioaccumulation
in fish. Molybdenum occurs naturally in soils at background concentrations ranging between 0.2 and 6 mg
kg-1, whereas metal-rich soils may contain 10–100 mg kg-1.
Metabolism
Not Available
storage
Color Code—Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Molybdenum must bestored to avoid contact with strong oxidizers (such as chlorine, bromine, and fluorine) since violent reactions occur.Store in tightly closed containers in a cool, well-ventilatedarea away from bromine, trifluoride, fluorine, chlorine trifluoride, and lead dioxide.
Shipping
UN3089 Metal powders, flammable, n.o.s., Hazard Class: 4.1; Labels: 4.1-Flammable solid.
Toxicity evaluation
Normally, enzymes containing molybdenum catalyze basic
metabolic reactions in the carbon, sulfur, and nitrogen cycles.
In plants, molybdenum acts as an enzyme activator for
nitrogen metabolism via reactions with nitrogenase, a nitrate
reductase. Consequently, molybdenum deficiency in legumes
produces effects similar to nitrogen deficiency. In mammals,
the types of reactions involving molybdenum-containing
enzymes include the transfer of oxygen atoms to or from the
electron pair of a substrate, and the oxidative hydroxylation of
aldehyde and aromatic compounds. Molybdenum is an
essential constituent of aldehyde oxidase, xanthine oxidase/
dehydrogenase, and sulfite oxidase, all of which catalyze
oxidation–reduction reactions. Molybdopterin maintains the
molybdenum atom to the active site of the protein in reactions
of the sulfur and carbon cycles. A deficiency of molybdopterin
has been associated with severe cerebral atrophy.
Molybdenosis or teart is a form of molybdenum toxicity
that produces a disease in ruminants similar to copper deficiency
in which trithiomolybdate ultimately alters the distribution
and elimination of copper. Signs of molybdenum
toxicity in animals include anemia, anorexia, profound diarrhea,
joint abnormalities, osteoporosis, hair discoloration,
reduced sexual activity, and death.
There is a paucity of data available on the human toxicity of
molybdenum. A goutlike syndrome and pneumoconiosis have
been associated with excessive concentrations of molybdenum,
but the inadequate design of the studies prevents an adequate
determination of the etiology of these effects.
Incompatibilities
Metallic Mo is a combustible solid in form of dust or powder and is potentially explosive. Dust or powder may form explosive mixture with air. Soluble compounds: alkali metals; sodium, potassium, molten magnesium. Insoluble compounds: Violent reaction with oxidizers, nitric acid; sulfuric acid. Forms explosive mixture with potassium nitrate. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Recovery is indicated whenever possible. Processes for recovery of Molybdenum from scrap, flue dusts, spent catalysts and other industrial wastes have been developed.
Properties of Molybdenum
| Melting point: | 2617 °C (lit.) |
| Boiling point: | 4612 °C (lit.) |
| Density | 10.3 g/mL at 25 °C (lit.) |
| refractive index | 2.81 (740 nm) |
| Flash point: | -23 °C |
| storage temp. | no restrictions. |
| solubility | H2O: soluble |
| form | wire |
| color | Gray |
| Specific Gravity | 10.2 |
| Resistivity | 5.0 μΩ-cm, 20°C |
| Water Solubility | Insoluble inwater. Soluble innitric acid andsulfuric acid. Slightly soluble inhydrochloric acid. |
| Sensitive | Air Sensitive |
| Merck | 13,6257 |
| Exposure limits | ACGIH: TWA 10 mg/m3; TWA 3 mg/m3 NIOSH: IDLH 5000 mg/m3 |
| Stability: | Stable. Powder is flammable. |
| CAS DataBase Reference | 7439-98-7(CAS DataBase Reference) |
| EPA Substance Registry System | Molybdenum (7439-98-7) |
Safety information for Molybdenum
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Health Hazard GHS08 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Molybdenum
Molybdenum manufacturer
Chaitanya Group of Industries
ARRAKIS INDUSTRIES LLP
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
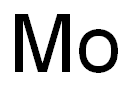

You may like
-
 Molybdenum, Mo 1000μg/mL CAS 7439-98-7View Details
Molybdenum, Mo 1000μg/mL CAS 7439-98-7View Details
7439-98-7 -
 Molybdenum, Mo 1000 μg/mL CAS 7439-98-7View Details
Molybdenum, Mo 1000 μg/mL CAS 7439-98-7View Details
7439-98-7 -
 Molybdenum, Mo 1000μg/mL CAS 7439-98-7View Details
Molybdenum, Mo 1000μg/mL CAS 7439-98-7View Details
7439-98-7 -
 Molybdenum wire, 0.375mm (0.015 in.) dia., Annealed, Temper: soft CAS 7439-98-7View Details
Molybdenum wire, 0.375mm (0.015 in.) dia., Annealed, Temper: soft CAS 7439-98-7View Details
7439-98-7 -
 Molybdenum plate, 4.76mm (0.188 in.) thick CAS 7439-98-7View Details
Molybdenum plate, 4.76mm (0.188 in.) thick CAS 7439-98-7View Details
7439-98-7 -
 Molybdenum rod, 5mm (0.2 in.) dia. CAS 7439-98-7View Details
Molybdenum rod, 5mm (0.2 in.) dia. CAS 7439-98-7View Details
7439-98-7 -
 Molybdenum wire, platinum coated, 0.457mm (0.018 in.) dia. CAS 7439-98-7View Details
Molybdenum wire, platinum coated, 0.457mm (0.018 in.) dia. CAS 7439-98-7View Details
7439-98-7 -
 Molybdenum standard CASView Details
Molybdenum standard CASView Details