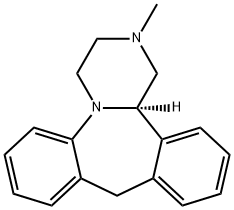
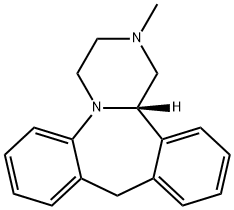
MIANSERIN
- CAS NO.:24219-97-4
- Empirical Formula: C18H20N2
- Molecular Weight: 264.36
- MDL number: MFCD00600076
- EINECS: 246-088-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
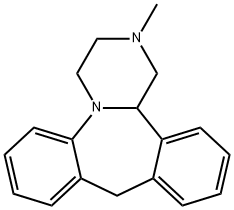
What is MIANSERIN?
Absorption
Absorbed following oral administration.
Toxicity
Oral rat LD50: 780mg/kg
Originator
Tolvin,Organon,W. Germany,1975
The Uses of MIANSERIN
Serotonin receptor antagonist. Antidepressant
Background
A tetracyclic compound with antidepressant effects. Mianserin was previously available internationally, however in most markets it has been phased out in favour of mirtazapine.
Indications
For the treatment of depression.
Definition
ChEBI: Mianserin is a dibenzoazepine (specifically 1,2,3,4,10,14b-hexahydrodibenzo[c,f]pyrazino[1,2-a]azepine) methyl-substituted on N-2. Closely related to (and now mostly superseded by) the tetracyclic antidepressant mirtazapinean, it is an atypical antidepressant used in the treatment of depression throughout Europe and elsewhere. It has a role as an antidepressant, a histamine agonist, a sedative, an alpha-adrenergic antagonist, an adrenergic uptake inhibitor, a serotonergic antagonist, a H1-receptor antagonist, an EC 3.4.21.26 (prolyl oligopeptidase) inhibitor and a geroprotector.
Manufacturing Process
(A) 25 g of 2-benzylaniline dissolved in 150 ml of benzene are cooled down in
an ice bath to 8°C. To this solution are added 15 ml of pyridine and after that
a solution of 15 ml of chloroacetyl chloride in 25 ml of benzene, maintaining
the temperature of the reaction mixture at 10° to 15°C. After stirring for 1
hour at room temperature 25 ml of water are added and the mixture is
shaken for 30 minutes. Next the mixture is sucked off and the benzene layer
separated. Then the benzene layer is washed successively with 2 N HCl, a
sodium carbonate solution and water. The extract dried on sodium sulfate is
evaporated and the residue crystallized together with the crystals obtained
already from benzene. Yield 18 g; MP 130° to 133°C.
(B) 40 g of N-chloroacetyl-2-benzylaniline are heated for 2 hours at 120°C
together with 50 ml of phosphorus oxychloride and 320 g of polyphosphoric
acid. Next the reaction mixture is poured on ice and extracted with benzene.
The extract is washed and dried on sodium sulfate and the benzene distilled
off. The product obtained (31g) yields after recrystallization 24 g of 6-
chloromethyl-morphanthridine of MP 136° to 137°C.
(C) 10 g of 6-chloromethyl-morphanthridine are passed into 150 ml of a
solution of methylamine in benzene (10%). After storage of the solution for
20 hours at 0° to 5°C the methylamine hydrochloride formed is sucked off
and the filtrate evaporated to dryness. There remains as residue 11 g of crude 6-methylaminomethyl-morphanthridine.
(D) 11 g of crude 6-methylaminomethyl-morphanthridine are dissolved in 50
ml of absolute ether. While cooling in ice 2.7 g of lithium aluminumhydride,
dissolved in 100 ml of absolute ether, are added. After boiling for 1 hour and
cooling down in ice 11 ml of water are added slowly dropwise while stirring.
After stirring for another 30 minutes at room temperature the mixture is
sucked off and the filtrate evaporated to obtain 11 g of crude 5,6-dihydro-6-
methylaminomethyl-morphanthridine in the form of a light yellow oil.
(E) 10 g of 5,6-dihydro-6-methylaminomethyl-morphanthridine are heated
slowly, in 30 minutes, from 100° to 160°C with 7 g of pure diethyloxalate and
after that from 160° to 180°C in 45 minutes. After cooling down the reaction
mixture is stirred with benzene. The crystals are sucked off and yield after
crystallization from dimethylformamide 9 g of 1,2-diketo-3(N)-methyl-
2,3,4,4a-tetrahydro-1H-pyrazino-[1,2-f]-morphanthridine of MP 245° to
247°C.
(F) 9 g of the diketo-pyrazino-morphanthridine compound obtained above are
reduced with diborane to give mianserin.
brand name
Athimil;Athymil;Bolvidon;Lantanon;Lerivon;Miansan;Norval;Org gb 94;Tolvin;Tolvon.
Therapeutic Function
Serotonin antagonist, Antihistaminic
World Health Organization (WHO)
Mianserin, a serotonin antagonist with antidepressant and antihistaminic activity, was introduced in 1975 for the treatment of depressive illness. Its use has since been associated with cases of severe blood dyscrasias, particularly in elderly patients, including agranulocytosis, leucopenia and granulocytopenia. Several drug regulatory authorities have reacted by stipulating that blood counts should be monitored regularly during the first few months of treatment and that administration should be discontinued immediately should any signs possibly indicative of dyscrasia develop.
Synthesis Reference(s)
Journal of Medicinal Chemistry, 13, p. 35, 1970 DOI: 10.1021/jm00295a010
Pharmacokinetics
Mianserin is a tetracyclic antidepressant that has antihistaminic and hypnosedative, but almost no anticholinergic, effect. It is a weak inhibitor of norepinephrine reuptake and strongly stimulates the release of norepinephrine. Interactions with serotonin receptors in the central nervous system have also been found. Its effect is usually noticeable after one to three weeks. Mianserin may cause drowsiness and hematological problems.
Metabolism
Hepatic.
Properties of MIANSERIN
| Boiling point: | 397.6°C (rough estimate) |
| Density | 1.0092 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | pKa 7.5(H2O,t =20.0) (Uncertain) |
Safety information for MIANSERIN
Computed Descriptors for MIANSERIN
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8