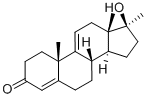
METHYLTESTOSTERONE
Synonym(s):17α-Methyl-4-androsten-17β-ol-3-one;17α-Methyltestosterone;17β-Hydroxy-17α-methyl-4-androsten-3-one;Mesterone;Methyltestosterone
- CAS NO.:67-81-2
- Empirical Formula: C25H38O2
- Molecular Weight: 370.57
- MDL number: MFCD00864406
- EINECS: 200-670-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-22 12:34:27
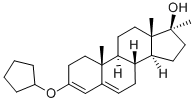
What is METHYLTESTOSTERONE?
Originator
Penmesterol ,Shanghai Lansheng
The Uses of METHYLTESTOSTERONE
Androgen.
Definition
ChEBI: Penmesterol is an androstanoid.
Manufacturing Process
A) 14 g of ethyl enolether of androstenedione, melting at 149°-151°C (obtained in a yield of 85% of the theoretical amount by treating androsteaedione with ethyl orthoformate), were added to a boiling solution of37 ml of cyclopentanol and 0.450 g of p-toluenesulfonic acid in 2.5 L of benzene. The mixture was distilled over an approximately 40 minute period, so that the ethanol, which evolved during the exchange reaction, was evaporated off completely.
Then 0.5 ml of pyridine was added to the remaining solution and the mixture was concentrated under vacuum to dryness. The residue, taken up with a mixture of methanolmethylene chloride containing a few drops of pyridine, gave 13.8 g of cyclopentyl enolether of androstenedione melting at 181°183°C. Yield about 85%.
B) The cyclopentyl enolether of androstenedione was converted to the corresponding cyclopentyl enolether of 17α-methyl testosterone as follows:In a 3-necked flask fitted with a dropping funnel, reflux condenser, stirrer and nitrogen inlet tube, there was placed a solution of 25 g of methyl magnesium bromide in 150 ml of ether. With stirring and under an atmosphere of nitrogen, a solution of 4.1 g of androstenedione 3-cyclopentyl enolether in 80 ml of anhydrous benzene was added slowly. The reaction mixture was refluxed for 1 hour and allowed to stand overnight at room temperature. The mixture was then treated with an aqueous solution of 30% ammonium chloride, the organic layer separated off, washed with water and dried over anhydrous sodium sulfate. The solvent was evaporated and the residue taken up with dilute methanol to yield 3.2 g of a white product. Crystallization from methanol containing few drops of pyridine give the pure 17α-methyltestosterone 3-cyclopentyl enolether; MP: 148-152°C; [α]D =-150° (dioxane).
brand name
Android (Valeant);Metandren (Novartis); Oreton (Schering); Testred (Valeant); Virilon (Star).
Therapeutic Function
Androgen
General Description
Methyltestosterone, 17β-hydroxy-17-methylandrost-4-en-3-one, is only about half asactive as testosterone (intramuscularly), but it has the greatadvantage of being orally active.
Properties of METHYLTESTOSTERONE
| Melting point: | 162-168 °C(lit.) |
| solubility | H2O: ≤0.5 mg/mL |
| form | solid (photosensitive) |
| color | white |
| CAS DataBase Reference | 67-81-2(CAS DataBase Reference) |
Safety information for METHYLTESTOSTERONE
Computed Descriptors for METHYLTESTOSTERONE
| InChIKey | ZUBDXGHKAAMAAA-CCSODHJYNA-N |
| SMILES | [C@]12(C)CC[C@]3([H])[C@@]4(C)CCC(OC5CCCC5)=CC4=CC[C@@]3([H])[C@]1([H])CC[C@@]2(O)C |&1:0,4,6,21,23,27,r| |
METHYLTESTOSTERONE manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 67-81-2 Penmestrol 98%View Details
67-81-2 Penmestrol 98%View Details
67-81-2 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1