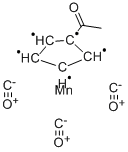
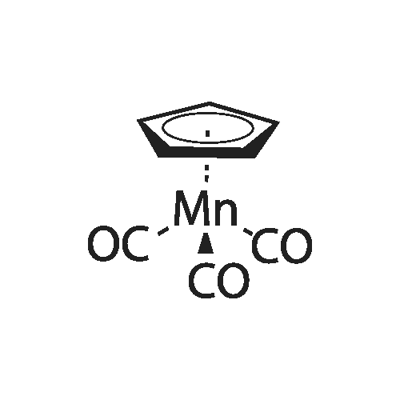
Methylcyclopentadienyl manganese tricarbonyl
Synonym(s):(Methylcyclopentadienyl)tricarbonylmanganese;Ethyl MMT;MCMT;MMT;Tricarbonyl(2-methylcyclopentadienyl)manganese
- CAS NO.:12108-13-3
- Empirical Formula: C9H7MnO35*
- Molecular Weight: 218.09
- MDL number: MFCD00001426
- EINECS: 235-166-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
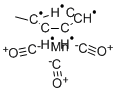
What is Methylcyclopentadienyl manganese tricarbonyl?
Description
Manganese, tricarbonyl methylcyclopentadienyl is a dark orange liquid. Faintly pleasant, herb-like odor. Molecular weight= 218.10;Boiling point=232℃; Freezing/Melting point=18℃; Flash point=74℃; 96℃. Hazard Identification (based on NFPA-704 M Rating System): Health 3, Flammability 0, Reactivity 0. Practically insoluble in water.
Chemical properties
An orange liquid with a pleasant odor. Slightly soluble in water and denser than water. May be toxic by inhalation, ingestion and/or skin absorption. Faintly pleasant, herb-like odor.
The Uses of Methylcyclopentadienyl manganese tricarbonyl
Antiknock fuel additive; dopant for Mn.
The Uses of Methylcyclopentadienyl manganese tricarbonyl
Methylcyclopentadienyl manganese tricarbonyl is used for doping of ZnSe nanocrystals
It can also be used as a reactant for:
Aldol addition reactions
Preparation of homo- and heteronuclear mixed biscarbene complexes with conjugated bithiophene units
Molecular fragmentation using shaped femtosecond laser pulses
Preparation of MnAs thin films grown on GaAs(001) by metalorganic vapour phase epitaxy (MOVPE)
The Uses of Methylcyclopentadienyl manganese tricarbonyl
Octane enhancer in gasoline; reduces smoke emissions from home, commercial, industrial, and marine burners
Definition
Methylcyclopentadienyl Manganese Tricarbonyl is a dark orange liquid with a faintly pleasant odor. It is used as a fuel additive to abate smoke and as a gasolinc additive in antiknock mixes.
Preparation
Into a reaction flask under nitrogen was placed 1.26 parts bis(methylcyclopentadienyl) manganese (91.8% pure), 0.93 parts manganous acetate, 0.78 parts tetrahydrofuran (THF) and 17.31 parts toluene. Over a period of 15 minutes, a solution of 1.24 parts triethyl aluminum (TEA) in 8.70 parts of toluene was added to the above mixture with a vigorous stirring (Al/Mn atom ratio 1/1, TEA/THF mole ratio 1/1). The solution darkened slightly. This solution of the intermediate complex was transferred under nitrogen to a stainless steel autoclave. The autoclave was sealed, pressurized twice to 300 psig with carbon monoxide and vented and finally pressurized with carbon monoxide to 600 psig and heated while stirring to 100° C. Carbon monoxide was added as needed to maintain 600 psig. After two hours at 100° C., the temperature was raised to 150° C. for 30 minutes. The autoclave was then cooled, vented and discharged. The mixture was hydrolyzed with 10% aqueous HCl. An equal volume of pentane was added to extract the MMT. The pentane phase was analyzed by gas chromoatograph (GC) using a pentadecane internal standard to show a yield of Methylcyclopentadienyl manganese tricarbonyl(MMT) based on manganese of 84% and, based on MCP, of 89%.
What are the applications of Application
Methylcyclopentadienyl Manganese Tricarbonyl is a compound used for doping of ZnSe nanocrystals
General Description
Methylcyclopentadienyl manganese tricarbonyl (hereinafter "MMT") is an antiknock agent for gasoline discovered in the fifties and sold commercially by Ethyl Corporation. It can be made by the reaction of carbon monoxide with bis(methylcyclopentadienyl) manganese referred to as "carbonylation".
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
METHYLCYCLOPENTADIENYLMANGANESE TRICARBONYL decomposes when exposed to light. METHYLCYCLOPENTADIENYLMANGANESE TRICARBONYL is stable in water.
Hazard
Toxic by ingestion, inhalation, and skinabsorption. Central nervous system impairment;lung, liver and kidney damage.
Health Hazard
In concentrated form METHYLCYCLOPENTADIENYLMANGANESE TRICARBONYL is highly toxic by all routes of exposure. Approximately 5-15 ml, when spilled on the hand and wrist of a worker, produced toxic effects within 3-5 minutes.
Fire Hazard
When heated to decomposition, METHYLCYCLOPENTADIENYLMANGANESE TRICARBONYL emits toxic fumes of carbon monoxide. Hazardous polymerization may not occur.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion, inhalation, skin contact, intravenous, and intraperitoneal routes. A slimn irritant. When heated to decomposition it emits toxic fumes of CO. See also MANGANESE COMPOUNDS and CARBONYLS.
Potential Exposure
MMT is used as an octane improver in unleaded gasoline, other distillate fuels, and fuel oils; as a smoke abater in fuels.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
storage
Color Code—Blue: Health Hazard/Poison: Store in a secure poison location. Prior to working with this chemical you should be trained on its proper handling and storage. Store in tightly closed containers in a cool, well-ventilated area away from oxidizers. Where possible, automatically pump liquid from drums or other storage containers to process containers.
Shipping
UN3281 Metal carbonyls, liquid n.o.s. Hazard class 6.1. Technical name required, Potential Inhalation Hazard (Special Provision 5).
Incompatibilities
Light causes decomposition. May be air-reactive. A strong reducing agent. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, halogens.
Waste Disposal
Generators of waste (equal to or greater than 100 kg/mo) containing this contaminant, EPA hazardous waste number N450, must conform to USEPA regulations for storage, transportation, treatment, and disposal of waste. Dispose of waste material as hazardous waste using a licensed disposal contractor to an approved landfill. Dispose of contents and container to an approved waste disposal plant. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. All federal, state, and local environmental regulations must be observed. Do not discharge into drains or sewers.
Properties of Methylcyclopentadienyl manganese tricarbonyl
| Melting point: | -1 °C (lit.) |
| Boiling point: | 232-233 °C (lit.) |
| Density | 1.38 g/mL at 25 °C (lit.) |
| vapor density | >1 (vs air) |
| vapor pressure | 0.05 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 205 °F |
| storage temp. | Poison room |
| form | liquid |
| color | yellow |
| Specific Gravity | 1.388 |
| Water Solubility | Insoluble in water. Soluble in hydrocarbon solvents, alcohols, ether, acetone |
| Sensitive | Air Sensitive |
| Merck | 14,6223 |
| Exposure limits | ACGIH: TWA 0.2 mg/m3 (Skin) OSHA: Ceiling 5 mg/m3 NIOSH: IDLH 500 mg/m3; TWA 0.2 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 12108-13-3(CAS DataBase Reference) |
| EPA Substance Registry System | Methylcyclopentadienyl manganese tricarbonyl (12108-13-3) |
Safety information for Methylcyclopentadienyl manganese tricarbonyl
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Methylcyclopentadienyl manganese tricarbonyl
| InChIKey | LYHJNAIHGFWRKM-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
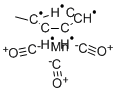

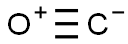

You may like
-
 Methylcyclopentadienylmanganese tricarbonyl CAS 12108-13-3View Details
Methylcyclopentadienylmanganese tricarbonyl CAS 12108-13-3View Details
12108-13-3 -
 (Methylcyclopentadienyl)manganese(I) tricarbonyl CAS 12108-13-3View Details
(Methylcyclopentadienyl)manganese(I) tricarbonyl CAS 12108-13-3View Details
12108-13-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1