Methylcyclopentane
Synonym(s):Methylcyclopentane
- CAS NO.:96-37-7
- Empirical Formula: C6H12
- Molecular Weight: 84.16
- MDL number: MFCD00001382
- EINECS: 202-503-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
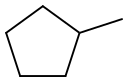
What is Methylcyclopentane?
Description
Methyl cyclopentane is a colorless liquid witha sweet gasoline-like odor. Molecular weight= 84.18;Boiling point = 72℃; Freezing/Melting point= - 142℃;Flash point= - 7℃; Autoignition temperature = 258℃.Explosive limits: LEL= 1.0%; UEL = 8.35%. HazardIdentification (based on NFPA-704 M Rating System):Health 2, Flammability 3, Reactivity 0.
Chemical properties
Methylcyclopentane is a colorless, flammable liquid with a sweet gasoline-like odor. An odor threshold concentration of 1.7 ppmv was reported by Nagata and Takeuchi (1990). Soluble in ether, miscible with alcohol and benzene, insoluble in water.
The Uses of Methylcyclopentane
Methylcyclopentane is used as an extractive solvent, an azeotropic distillation agent, and in organic synthesis. [Hawley]
The Uses of Methylcyclopentane
Methylcyclopentane can undergo ring opening or ring enlargement to yield various hydrocarbons including branched and unbranched hexanes, cyclohexane and benzene.
Definition
ChEBI: Methylcyclopentane is a cycloalkane that is cyclopentane substituted by a single methyl group. It has a role as a human metabolite and a plant metabolite. It is a cycloalkane and a volatile organic compound. It derives from a hydride of a cyclopentane.
Preparation
Methylcyclopentane mainly exists in industrial hexane, accounting for about 5%. However, because its boiling point is close to that of n-hexane (68.74°C), it is difficult to completely separate it by general rectification methods. Therefore, methylcyclopentane with a purity of more than 99% can be obtained by azeotropic distillation with methanol.
General Description
Methylcyclopentane appears as a colorless liquid. Insoluble in water and less dense than water. Flash point near 20 °F. Very dangerous fire risk. Vapors may be narcotic and irritating. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Methylcyclopentane can react vigorously with oxidizers. (NTP, 1992).
Hazard
Flammable, dangerous fire and explosionrisk. May be irritant and narcotic.
Health Hazard
Inhalation causes dizziness, nausea, and vomiting; concentrated vapor may cause unconsciousness and collapse. Liquid causes irritation of eyes and mild irritation of skin if allowed to remain. Ingestion causes irritation of stomach. Aspiration causes severe lung irritation, rapidly developing pulmonary edema, and central nervous system excitement followed by depression.
Fire Hazard
Behavior in Fire: Vapor is heavier than air and may travel a considerable distance to a source of ignition and flash back.
Safety Profile
Mildly toxic by inhalation. Probably irritating and narcotic in high concentration. Very dangerous fire hazard when exposed to heat, flame, or oxidizers. Can react vigorously with oxidizing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
Methylcyclopentane, occurs in various petroleums.It is readily formed by the isomerization of cyclohexane with aluminum chloride.Hydrogen and Ni at 460℃ also convert cyclohexane to methylcyclopentane. It is more readily oxidized than cyclopentane, presumably because of the tertiary H. Heating with dil. nitric acid replaces this H by a nitro group.
Potential Exposure
This material is used as a solvent; as a fuel; and in chemical synthesis.
First aid
If this chemical gets into the eyes, remove any con-tact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medi-cal attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin res-cue breathing (using universal precautions, including resusci-tation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention.Give large quantities of water and induce vomiting. Do notmake an unconscious person vomit. Medical observation isrecommended for 24- -48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonaryedema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
Source
A constituent in gasoline. Harley et al. (2000) analyzed the headspace vapors of three
grades of unleaded gasoline where ethanol was added to replace methyl tert-butyl ether. The
gasoline vapor concentrations of methylcyclopentane in the headspace were 2.7 wt % for regular
grade, 2.6 wt % for mid-grade, and 2.6 wt % for premium grade.
California Phase II reformulated gasoline contained methylcyclopentane at a concentration of
26.2 g/kg. Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without
catalytic converters were 4.32 and 604 mg/km, respectively (Schauer et al., 2002).
Environmental Fate
Photolytic. A photooxidation rate constant of 7.0 x 10-12 cm3/molecule?sec was reported for the
reaction of methylcyclopentane and OH radicals in the atmosphere (Atkinson, 1990).
Chemical/Physical. Complete combustion in air produces carbon dioxide and water vapor.
Methylcyclopentane will not hydrolyze because it does not contain a hydrolyzable functional
group.
At elevated temperatures, rupture of the ring occurs and 1-propene is produced in a 40% yield.
Other products include hydrogen and cyclic mono- and diolefins (Rice and Murphy, 1942).
Solubility in water
In methanol, g/L: 380 at 5 °C, 415 at 10 °C, 500 at 15 °C, 595 at 20 °C, 740 at 25 °C, 1,100 at 30 °C. Miscible at higher temperatures (Kiser et al., 1961).
storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist. Methylcyclopentane must be stored to avoid contact with oxidizers(such as perchlorates, peroxides, permanganates, chloratesand nitrates) and strong acids, since violent reactions occur.Sources of ignition, such as smoking and open flames, areprohibited where methyl cyclopentane is handled, used, orstored. Metal containers involving the transfer of 5 gallonsor more of methyl cyclopentane should be grounded andbonded. Drums must be equipped with self-closing valves,pressure vacuum bungs, and flame arresters. Use only nonsparking tools and equipment, especially when opening andclosing containers of methyl cyclopentane. Wherevermethyl cyclopentane is used, handled, manufactured, orstored, use explosion-proof electrical equipment andfittings.
Shipping
UN2298 Methyl cyclo pentane, Hazard Class: 3; Labels: 3-Flammable liquid
Purification Methods
Purification procedures include passage through columns of silica gel (prepared by heating in nitrogen to 350o prior to use) and activated basic alumina, distillation from sodium-potassium alloy, and azeotropic distillation with MeOH, followed by washing out the methanol with water, drying and distilling. It can be stored with CaH2 or sodium. [Vogel J Chem Soc 1331 1938, Beilstein 5 III 55, 5 IV 84.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Methylcyclopentane
| Melting point: | -142.4 °C |
| Boiling point: | 72 °C(lit.) |
| Density | 0.749 g/mL at 25 °C(lit.) |
| vapor pressure | 232.8 mm Hg ( 37.7 °C) |
| refractive index | n |
| Flash point: | −11 °F |
| storage temp. | Store below +30°C. |
| solubility | In methanol, g/L: 380 at 5 °C, 415 at 10 °C, 500 at 15 °C, 595 at 20 °C, 740 at 25 °C, 1,100 at 30
°C. Miscible at higher temperatures (Kiser et al., 1961). |
| form | Adhering Crystals |
| color | White |
| Odor | Like gasoline. |
| explosive limit | 8.35% |
| Odor Threshold | 1.7ppm |
| Water Solubility | 41.8mg/L(25 ºC) |
| BRN | 1900214 |
| Henry's Law Constant | 0.362 atm?m3/mol at 25 °C (Hine and Mookerjee, 1975) |
| Dielectric constant | 2.0(20℃) |
| CAS DataBase Reference | 96-37-7(CAS DataBase Reference) |
| EPA Substance Registry System | Methylcyclopentane (96-37-7) |
Safety information for Methylcyclopentane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Methylcyclopentane
Methylcyclopentane manufacturer
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran
You may like
-
 Methylcyclopentane, 97% 99%View Details
Methylcyclopentane, 97% 99%View Details
96-37-7 -
 Methylcyclopentane CAS 96-37-7View Details
Methylcyclopentane CAS 96-37-7View Details
96-37-7 -
 Methylcyclopentane 95% CAS 96-37-7View Details
Methylcyclopentane 95% CAS 96-37-7View Details
96-37-7 -
 Methyl cyclopentane CAS 96-37-7View Details
Methyl cyclopentane CAS 96-37-7View Details
96-37-7 -
 Methylcyclopentane CAS 96-37-7View Details
Methylcyclopentane CAS 96-37-7View Details
96-37-7 -
 Methylcyclopentane CAS 96-37-7View Details
Methylcyclopentane CAS 96-37-7View Details
96-37-7 -
 Methylcyclopentane CAS 96-37-7View Details
Methylcyclopentane CAS 96-37-7View Details
96-37-7 -
 Methylcyclopentane CAS 96-37-7View Details
Methylcyclopentane CAS 96-37-7View Details
96-37-7
