Methyl thioglycolate
Synonym(s):Methyl mercaptoacetate
- CAS NO.:2365-48-2
- Empirical Formula: C3H6O2S
- Molecular Weight: 106.14
- MDL number: MFCD00004873
- EINECS: 219-121-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:15
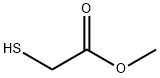
What is Methyl thioglycolate?
Chemical properties
CLEAR COLOURLESS LIQUID
The Uses of Methyl thioglycolate
Methyl Thioglycolate is an derivative of Thioglycolic Acid (T350760), an organic compound containing both a thiol and a carboxylic acid functional groups. Thioglycolic Acid and its derivatives are oft en used in organic synthesis as a nucleophile in thioglycolysis reactions and is also used as a S transfer agent for sulfonyl chloride synthesis. Methyl Thioglycolate have been studied for the its inf luence on neocarzinostatin activation and expression of DNA damage.
The Uses of Methyl thioglycolate
Methyl thioglycolate was used in the preparation of 3-carbomethoxy-4- oxotetrahydrothiopyran, 2- and 4-carbomethoxy-3-oxotetrahydrothiophene. It is used to prepare methyl thioglycolate and aminoethanethiol conjugated gold nanorods.
What are the applications of Application
Methyl thioglycolate is A thiol organocatalyst
What are the applications of Application
Methyl thioglycolate is a chemical compound that is used in the pharmaceutical industry as an intermediate. It has been used to synthesize anti-inflammatory drugs, such as aspirin and ibuprofen, and also for the treatment of autoimmune diseases. Methyl thioglycolate binds to amino acids on the surface of bacteria, which prevents them from functioning properly. This binding causes the cells to stop growing and die.
Definition
Methyl thioglycolate are widely used in free-radical photoinitiated polymerization reactions. In particular, the use of methyl thioglycolate (MTG), CH3OC(O)CH2SH, results in greater photopolymerization reaction rates, attributed to a weakening of the sulfur–hydrogen bond by hydrogen bonding of the thiol group. MTG constitutes the smaller exponent of the series of CH3OC(O)(CH2)nSH compounds, reported as intermediated in the organic sulfur cycle in marine environments, produced by marine phytoplankton.
Production Methods
Adding thioglycollic acid with purity of between 70.0 and 96.0 percent and methanol with purity of between 95.0 and 99.9 percent into a reaction device according to the molar ratio of 1:1.3-4.0; subsequently, adding an activating agent into the reaction device, stirring and heating for reaction; and after the reaction is finished, carrying out separatory distillation on reaction products to obtain the methyl thioglycolate. The activating agent is a p-toluenesulfonic acid methanol solution with a mass concentration of between 20 and 30 percent, and the use amount is 0.5 to 1.0 percent of the total mass of relative input materials.
General Description
Methyl thioglycolate reacts with nonprotein component of the antitumor antibiotic neocarzinostatin to form 1:1 adduct. It reacts with isothiocyanate to form Rhodanine.
Properties of Methyl thioglycolate
| Melting point: | -24 °C |
| Boiling point: | 42-43 °C/10 mmHg (lit.) |
| Density | 1.187 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 86 °F |
| storage temp. | Store below +30°C. |
| solubility | 40g/l |
| pka | 8.04±0.10(Predicted) |
| form | Liquid |
| color | Clear colorless |
| Water Solubility | 40 g/L (20 ºC) |
| Sensitive | Air Sensitive |
| BRN | 506259 |
| CAS DataBase Reference | 2365-48-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, mercapto-, methyl ester(2365-48-2) |
| EPA Substance Registry System | Acetic acid, mercapto-, methyl ester (2365-48-2) |
Safety information for Methyl thioglycolate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methyl thioglycolate
| InChIKey | MKIJJIMOAABWGF-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 2365-48-2 Mercaptoacetic acid methyl ester 98%View Details
2365-48-2 Mercaptoacetic acid methyl ester 98%View Details
2365-48-2 -
 Methyl mercaptoacetate CAS 2365-48-2View Details
Methyl mercaptoacetate CAS 2365-48-2View Details
2365-48-2 -
 Methyl Thioglycolate CAS 2365-48-2View Details
Methyl Thioglycolate CAS 2365-48-2View Details
2365-48-2 -
 Methyl thioglycolate CAS 2365-48-2View Details
Methyl thioglycolate CAS 2365-48-2View Details
2365-48-2 -
 Methyl mercaptoacetate 98% (GC) CAS 2365-48-2View Details
Methyl mercaptoacetate 98% (GC) CAS 2365-48-2View Details
2365-48-2 -
 Methyl thioglycolate CAS 2365-48-2View Details
Methyl thioglycolate CAS 2365-48-2View Details
2365-48-2 -
 Methyl MercaptoacetateView Details
Methyl MercaptoacetateView Details
2365-48-2 -
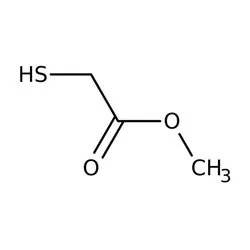 Padmaja Chemical Methyl Thioglycolate, Chemical Formula: C3H6O2SView Details
Padmaja Chemical Methyl Thioglycolate, Chemical Formula: C3H6O2SView Details
2365-48-2
