Methyl methacrylate
Synonym(s):EPN;MME;Neprilysin;Atriopeptidase;Common acute lymphocytic leukemia antigen
- CAS NO.:80-62-6
- Empirical Formula: C5H8O2
- Molecular Weight: 100.12
- MDL number: MFCD00008587
- EINECS: 201-297-1
- Update Date: 2025-10-29 10:02:07
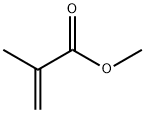
What is Methyl methacrylate?
Description
Methyl methacrylate (MMA) is an unsaturated ester that has several uses in polymer manufacturing. It is a clear liquid with a characteristic ester odor.
There are several synthetic routes to MMA. The most widely used are a three-step sequence that begins with acetone and HCN and proceeds through acetone cyanohydrin; and a two-step process that begins with the reaction of ethylene and methanol to produce methyl propionate.
By far the greatest volume of MMA is polymerized to the homopolymer poly(methyl methacrylate) (PMMA). The polymer is a clear plastic that is known by the familiar trade names Lucite and Plexiglas.
MMA is also copolymerized with styrene and butadiene to make an additive to poly(vinyl chloride) (PVC) that improves its properties. MMA is also transesterified to make specialty methacrylate monomers.
The Uses of Methyl methacrylate
Methyl methacrylatec is used in acrylic bone cements used in orthopedic surgery; in the production of acrylic polymers, polymethylmethacrylate and copolymers used in acrylic surface coatings; in the manufaeture of emulsion polymers; in the modification of unsaturated polyester resins; in the production of higher methacrylate, acrylic fibers, acrylic film, inks, radiation-polymerized impregnants for wood, and solvent-based adhesives and binders; as an impact modifier of PVC; in medicinal spray adhesives; in nonirritant bandage solvents; in dental technology as ceramic filler or cement; to coat corneal contact lenses; in intraocular lenses, artificial nails, and hearing aids; as a monomer for polymethaerylate resins; in the impregnation of concretc.
What are the applications of Application
Methyl methacrylate is the methyl ester of methacrylic acid
Definition
ChEBI: Methyl methacrylate is an enoate ester having methacrylic acid as the carboxylic acid component and methanol as the alcohol component. It has a role as an allergen and a polymerisation monomer. It is an enoate ester and a methyl ester. It is functionally related to a methacrylic acid.
Synthesis Reference(s)
Journal of the American Chemical Society, 70, p. 1153, 1948 DOI: 10.1021/ja01183a082
The Journal of Organic Chemistry, 33, p. 2525, 1968 DOI: 10.1021/jo01270a082
Flammability and Explosibility
Flammable
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Structure
Methyl methacrylate (MMA) belongs to the Cs point group. It has a twofold reaction path degeneracy due to its two equivalent faces which can be attacked by the propagating radical. Moreover, MMA has two isomers – s-cis and s-trans, as defined by rotation around the single bond of the methacrylate group.
Health effect
In general, methyl methacrylate has low acute toxicity, but it is a mild skin irritant in humans, and it is not considered a carcinogenic agent.
Toxicity evaluation
The mitochondria are regarded as the main intracellular target of MMA. If isolated rat liver mitochondria are incubated with MMA, oxygen consumption increases. This is the result of an uncoupling of the mitochondrial respiratory chain, as seen from the expected influence on state 4 and state 3 respiration. State 4 respiration is stimulated. As has been reported for organic solvents, MMA attacks complex I of the respiratory chain close to the rotenonebinding site. This means that substrates which are oxidized in conjunction with nicotinamide adenine dinucleotide inhibit the flow of electrons and thus also ATP synthesis. Unlike classical uncouplers, MMA stimulates the Mg2+- dependent ATPase bound to the inner mitochondrial membrane. Structural changes in the inner membrane, as found with nonionic detergents, were observed by electron micro scopy. The release of enzymes indicates disintegration of the membrane.
Properties of Methyl methacrylate
| Melting point: | -48 °C (lit.) |
| Boiling point: | 100 °C (lit.) |
| Density | 0.936 g/mL at 25 °C (lit.) |
| vapor density | 3.5 (vs air) |
| vapor pressure | 29 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 4002 | METHYL 2-METHYL-2-PROPENOATE |
| Flash point: | 50 °F |
| storage temp. | 2-8°C |
| solubility | 15g/l |
| form | Crystalline Powder or Crystals |
| appearance | colorless liquid |
| color | White to pale yellow |
| Odor | at 0.10 % in dipropylene glycol. acrylic aromatic fruity |
| explosive limit | 2.1-12.5%(V) |
| Odor Threshold | 0.21ppm |
| Water Solubility | 15.9 g/L (20 ºC) |
| Merck | 14,5941 |
| JECFA Number | 1834 |
| BRN | 605459 |
| Henry's Law Constant | 2.46 x 10-4 atm?m3/mol at 20 °C (approximate - calculated from water solubility and vapor pressure) |
| Exposure limits | NIOSH REL: TWA 100 ppm (410 mg/m3), IDLH 1,000 ppm; OSHA PEL:
TWA 100 ppm; ACGIH TLV: TWA 100 ppm with intended TWA and STEL values of 50 and
100 ppm, respectively. |
| Dielectric constant | 2.9(20℃) |
| Stability: | Volatile |
| CAS DataBase Reference | 80-62-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propenoic acid, 2-methyl-, methyl ester(80-62-6) |
| IARC | 3 (Vol. Sup 7, 60) 1994 |
| EPA Substance Registry System | Methyl methacrylate (80-62-6) |
Safety information for Methyl methacrylate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H317:Sensitisation, Skin H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Methyl methacrylate
| InChIKey | VVQNEPGJFQJSBK-UHFFFAOYSA-N |
Methyl methacrylate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

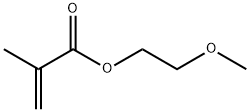
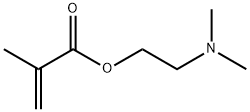
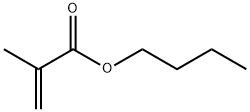
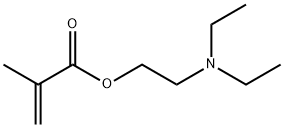
You may like
-
 Methyl methacrylate, Stabilized CAS 80-62-6View Details
Methyl methacrylate, Stabilized CAS 80-62-6View Details
80-62-6 -
 Methyl Methacrylate CAS 80-62-6View Details
Methyl Methacrylate CAS 80-62-6View Details
80-62-6 -
 Methyl Methacrylate CASView Details
Methyl Methacrylate CASView Details -
 Methyl Methacrylate CASView Details
Methyl Methacrylate CASView Details -
 85%Methyl Methacrylate Liquid CAS 80-62-6View Details
85%Methyl Methacrylate Liquid CAS 80-62-6View Details
80-62-6 -
 Methyl methacryalate 99% CAS 80-62-6View Details
Methyl methacryalate 99% CAS 80-62-6View Details
80-62-6 -
 Methyl Methacrylate (MMA), Liquid, 99 %View Details
Methyl Methacrylate (MMA), Liquid, 99 %View Details
80-62-6 -
 Methyl Methacrylic AcidView Details
Methyl Methacrylic AcidView Details
80-62-6
