Glycidyl methacrylate
Synonym(s):2,3-Epoxypropyl methacrylate;GMA;Methacrylic acid 2,3-epoxypropyl ester
- CAS NO.:106-91-2
- Empirical Formula: C7H10O3
- Molecular Weight: 142.15
- MDL number: MFCD00005137
- EINECS: 203-441-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
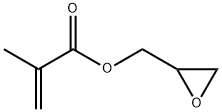
What is Glycidyl methacrylate?
Description
Glycidyl Methacrylate (GMA) is a clear, colorless liquid with a potent ester and fruity odor. It comprises a polymerizable methacrylate functional group on one end and a reactive epoxy group on the other. It is slightly miscible with water, soluble in most organic solvents, and has relatively low volatility. Its vapor is heavier than air. It copolymerizes readily with various functional groups, such as phenols, ketones, acids, and amines. The added epoxy group imparts durability, mechanical strength, optical transparency, and adhesiveness.
Chemical properties
Glycidyl methacrylate (GMA) is a colorless liquid with a fruity odor. Floats on water. (USCG, 1999)

Glycidyl methacrylate is a polyfunctional monomer. It acts as an adhesion promoting crosslinking co-monomer for acrylic and vinyl resins. It is also a reactive colorless diluent. GMA is soluble in ethanol, acetone, diethyl ether, benzene.
The Uses of Glycidyl methacrylate
Glycidyl methacrylate (GMA) is a common monomer used in the creation of epoxy resins. It is used to provide epoxy functionalization to polyolefins and other acrylate resins.
Glycidyl methacrylate is used in the production of polymer coatings and finishes, adhesives, plastics and elastomers.
Glycidyl methacrylate dextran has been reported to be used as a biocompatible hydrogel. In situ polymerization of GMA with trimethylolpropane trimethacrylate to form macroporous sorbents has also been reported. GMA may also be grafted onto polypropylene.
The Uses of Glycidyl methacrylate
Glycidyl methacrylate is commonly used as a monomer for the preparation of epoxy resins. It is also used to provide epoxy functionalization to polyolefins and other acrylate resins. Further, it is also useful as an epoxy resin additive for paint coating formulations and adhesive applications.
Preparation
Glycidyl methacrylate is produced from methacrylic acid and glycidol. Glycidol contain both epoxide and alcohol functional groups.It is an enoate ester and an epoxide. It derives from a methacrylic acid and a glycidol.
Definition
ChEBI: Glycidyl methacrylate is an enoate ester obtained by formal condensation of the carboxy group of methacrylic acid with the hydroxy group of glycidol. It is an enoate ester and an epoxide. It is functionally related to a methacrylic acid and a glycidol.
Reactions
Glycidyl methacrylate (GMA) is a kind of functional monomer. Its active vinyl and epoxy groups could be grafted with polyolefin and reacted with the polar groups such as amine, hydroxyl and carboxyl group, respectively. It can also undergo polymerization to form poly (glycidyl methacrylate).
Synthesis Reference(s)
Tetrahedron, 48, p. 5099, 1992 DOI: 10.1016/S0040-4020(01)90120-6
Synthesis, p. 1019, 1986 DOI: 10.1055/s-1986-31856
General Description
Colorless liquid with a fruity odor. Floats on water.
Air & Water Reactions
Flammable. Slightly water soluble.
Reactivity Profile
Epoxides, such as Glycidyl methacrylate, are highly reactive. They polymerize in the presence of catalysts or when heated. These polymerization reactions can be violent. Compounds in this group react with acids, bases, and oxidizing and reducing agents. They react, possibly violently with water in the presence of acid and other catalysts.
Health Hazard
The liquid irritates eyes about as much as soap. Prolonged contact with skin produces irritation and dermatitis.
Fire Hazard
Special Hazards of Combustion Products: Irritating vapors are generated when heated
Flammability and Explosibility
Flammable
Properties of Glycidyl methacrylate
| Melting point: | -52°C |
| Boiling point: | 189 °C(lit.) |
| Density | 1.075 g/mL at 20 °C |
| vapor pressure | 4.2hPa at 25℃ |
| refractive index | n |
| Flash point: | 169 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Liquid |
| color | Clear |
| Water Solubility | 0.5-1.0 g/100 mL at 20 ºC |
| BRN | 2506 |
| Stability: | Unstable. Light sensitive. May be stabilized with around 100ppm MEHQ. Refrigerate. |
| CAS DataBase Reference | 106-91-2(CAS DataBase Reference) |
| IARC | 2A (Vol. 125) In prep. |
| NIST Chemistry Reference | 2-Propenoic acid, 2-methyl-, oxiranylmethyl ester(106-91-2) |
| EPA Substance Registry System | Glycidyl methacrylate (106-91-2) |
Safety information for Glycidyl methacrylate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H311:Acute toxicity,dermal H314:Skin corrosion/irritation H317:Sensitisation, Skin H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H341:Germ cell mutagenicity H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Glycidyl methacrylate
| InChIKey | VOZRXNHHFUQHIL-UHFFFAOYSA-N |
Glycidyl methacrylate manufacturer
New Products
(R)-1-Boc-3-hydroxypyrrolidine Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

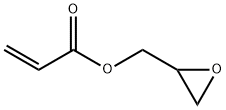
You may like
-
 Glycidyl methacrylate, 97% CAS 106-91-2View Details
Glycidyl methacrylate, 97% CAS 106-91-2View Details
106-91-2 -
 Glycidyl methacrylate, stabilized with 100ppm 4-methoxyphenol CAS 106-91-2View Details
Glycidyl methacrylate, stabilized with 100ppm 4-methoxyphenol CAS 106-91-2View Details
106-91-2 -
 Glycidyl Methacrylate (stabilized with MEHQ) CAS 106-91-2View Details
Glycidyl Methacrylate (stabilized with MEHQ) CAS 106-91-2View Details
106-91-2 -
 Glycidyl Methacrylate pure CAS 106-91-2View Details
Glycidyl Methacrylate pure CAS 106-91-2View Details
106-91-2 -
 Glycidyl methacrylate CAS 106-91-2View Details
Glycidyl methacrylate CAS 106-91-2View Details
106-91-2 -
 Glycidyl methacrylate CAS 106-91-2View Details
Glycidyl methacrylate CAS 106-91-2View Details
106-91-2 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4
