Methyl carbamate
Synonym(s):p110-alpha;p55-gamma;p85-alpha;Urethylane
- CAS NO.:598-55-0
- Empirical Formula: C2H5NO2
- Molecular Weight: 75.07
- MDL number: MFCD00007964
- EINECS: 209-939-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-21 20:54:13
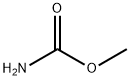
What is Methyl carbamate?
Chemical properties
WHITE ADHERING CRYSTALS
The Uses of Methyl carbamate
Methyl carbamate was used in the synthesis of protected aminocyclopropanes.
Definition
ChEBI: Methyl carbamate is a carbamate ester resulting from the formal condensation of the carboxy group of carbamic acid with methanol.
Preparation
Methyl carbamate was synthesized by the following preparation. MDI (5.00 g,0.020mol) and 100 ml of dried acetone were weighed into a Ng-filled three-neck flask.Methanol (5 g, an excess amount) is added dropwise to the stirred MDI solution in the flask,and then the solution was heated to 56 C. After 24 hrs, the reaction mixture was cooled down to room temperature and evaporated to give a crude solid at about 100% yield. The following recrystallization procedure gave 3.1 g of a white solid (0.012 mol,60% yield). The disappearance of FT-IR stretching band of the isocyanate group (-N=C=O, 2280 cm') confirmed that the reaction was complete.
General Description
White crystals.
Air & Water Reactions
Water soluble.
Reactivity Profile
Methyl carbamate is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates.
Fire Hazard
Flash point data for Methyl carbamate are not available; however, Methyl carbamate is probably combustible.
Safety Profile
Poison by ingestion and intraperitoneal routes. Questionable carcinogen with experimental carcinogenic and tumorigenic data. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx. See also CARBAMATES.
Purification Methods
Crystallise the carbamate from *benzene or distil it. [Beilstein 3 H 21.]
Properties of Methyl carbamate
| Melting point: | 56-58 °C (lit.) |
| Boiling point: | 176-177 °C (lit.) |
| Density | 1,14 g/cm3 |
| refractive index | 1.4125 |
| Flash point: | 93 °C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | 700 g/L (20°C) |
| form | Adhering Crystals |
| pka | 13.37±0.50(Predicted) |
| color | White |
| PH | 6-8 (50g/l, H2O, 20℃) |
| Water Solubility | 700 g/L (20 ºC) |
| Sensitive | Moisture Sensitive |
| Merck | 14,6036 |
| BRN | 635779 |
| CAS DataBase Reference | 598-55-0(CAS DataBase Reference) |
| IARC | 3 (Vol. 12, Sup 7) 1987 |
| NIST Chemistry Reference | Carbamic acid, methyl ester(598-55-0) |
| EPA Substance Registry System | Methyl carbamate (598-55-0) |
Safety information for Methyl carbamate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Methyl carbamate
Abamectin manufacturer
Greenovel life sciences Pvt Ltd
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran






You may like
-
 598-55-0 Carbamic acid methyl ester 99%View Details
598-55-0 Carbamic acid methyl ester 99%View Details
598-55-0 -
 Methyl carbamate 98% CAS 598-55-0View Details
Methyl carbamate 98% CAS 598-55-0View Details
598-55-0 -
 Methyl Carbamate CAS 598-55-0View Details
Methyl Carbamate CAS 598-55-0View Details
598-55-0 -
 Methyl carbamate CAS 598-55-0View Details
Methyl carbamate CAS 598-55-0View Details
598-55-0 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4