Methyl butyrate
- CAS NO.:623-42-7
- Empirical Formula: C5H10O2
- Molecular Weight: 102.13
- MDL number: MFCD00009391
- EINECS: 210-792-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
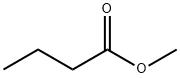
What is Methyl butyrate?
Description
Methyl butyrate, also known as methyl butanoate , is an ester with a fruity odor pineapple, apple, and strawberry. At room temperature, it is a colorless liquid with low solubility in water, upon which it floats to form an oily layer. Although it is flammable, it has a relatively low vapor pressure ( 40 mmHg at 30°C), so it can be safely handled at room temperature without special safety precautions.
Chemical properties
Methyl butyrate is colourless liquid that has an apple-like odor and a corresponding sweet taste that is not very powerful. Below 100 ppm, it may have a banana-pineapple flavor. Miscible in ethanol and ether, slightly soluble in water (1:60). May be prepared from methyl alcohol and butyric acid in the presence of concentrated H2S04.
Occurrence
Metyhl butyrate occurs naturally in pineapple oil, round grapefruit juice, apple juice, kiwi and mushrooms. It is used as a flavor ingredient in fruit and rum flavoring for beverages, ice cream, candy, and baked goods.
The Uses of Methyl butyrate
Methyl butyrate is used as a solvent for ethylcellulose and nitrocellulose resins. It is also used in lacquers and perfumes and in the manufacture of rum and fruit flavors.
Preparation
Methyl butyrate is produced from methyl alcohol and butyric acid in the presence of concentrated sulfuric acid. The synthesis of methyl butyrate by the reaction between butyric acid and methanol with acidic ion exchange resin as a catalyst in presence of ultrasound irradiation was successfully carried out.
Definition
ChEBI: Methyl butyrate is a fatty acid ester.
What are the applications of Application
Methyl butyrate is an important raw material and intermediate used in organic synthesis, pharmaceuticals, agrochemicals and dyestuff fields.
Aroma threshold values
Detection: 1 to 43 ppb. Aroma characteristics at 1.0%: pungent, ethereal, fruity, fumey and fusel with a fermented, cultured, creamy undernote.
Taste threshold values
Taste characteristics at 10 ppm: fruity and apple-like with a sweet almost buttery, nutty and creamy nuance, fusel-like, impacting, and estry with a cultured dairy, acidic depth.
Synthesis Reference(s)
Tetrahedron Letters, 29, p. 1759, 1988 DOI: 10.1016/S0040-4039(00)82035-3
General Description
Methyl butyrate appears as a clear colorless liquid. Flash point 57°F. Less dense than water and slightly soluble in water. Hence floats on water. Vapors heavier than air.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
Methyl butyrate reacts exothermically with acids to generate alcohols and carboxylic acids. Strong oxidizing acids may cause a reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by interaction with basic or caustic solutions. Flammable hydrogen is generated by mixing with alkali metals and hydrides .
Hazard
Flammable, dangerous fire risk.
Health Hazard
Irritating to the eyes, nose, throat, upper respiratory tract, and skin.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Industrial uses
Methyl butyrate is occasionally used as a masking
agent in industrial perfumes especially where
the problem is to mask very volatile malodors,
etc. The type of odor is particularly suitable
to cover sulfuraceous odors.
It is, however, much more widely used in
flavor compositions for imitation Apple,
Banana, Pineapple, Melon, Peach, Rum and
Arak, and in various fruit complexes.
The concentration used is equivalent to
about 20 to 100 ppm in the finished consumer
product.
Safety Profile
Moderately toxic by ingestion and skin contact. A skin irritant. A very dangerous fire hazard when exposed to heat, flame, or oxidizers. Can react vigorously with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS.
Potential Exposure
Methyl butyrate, one of the odorous compound present in different types of adhesive (hotmelt, vinyl acetate ethylene, starch, polyvinyl acetate and acrylic) used in food packaging, was identified by GC-O-MS (gas chromatography–mass spectrometry–olfactometry).
Carcinogenicity
Not listed by ACGIH, California Proposition 65, IARC, NTP, or OSHA.
Purification Methods
Treat the ester with anhydrous CuSO4, then distil it under dry nitrogen. [Beilstein 2 IV 786.]
References
[1] Ruth Winter, A Consumer's Dictionary of Food Additives, 7th Edition, 2009
[2] Laurent Ducry and Dominique M. Roberge, Dibal-H Reduction of Methyl Butyrate into Butyraldehyde using Microreactors, Organic Process Research & Development, 2008, vol. 12, 163-167
Properties of Methyl butyrate
| Melting point: | -85--84°C |
| Boiling point: | 102-103 °C (lit.) |
| Density | 0.898 g/mL at 25 °C (lit.) |
| vapor density | 3.5 (vs air) |
| vapor pressure | 40 mm Hg ( 30 °C) |
| refractive index | n |
| FEMA | 2693 | METHYL BUTYRATE |
| Flash point: | 53 °F |
| storage temp. | Flammables area |
| solubility | water: soluble60 part |
| form | Liquid |
| color | Clear colorless to very slightly yellow |
| Odor | at 10.00 % in dipropylene glycol. fruity apple sweet banana pineapple |
| Odor Threshold | 0.0071ppm |
| explosive limit | 1.6%(V) |
| Water Solubility | Slightly soluble in water. |
| Merck | 14,6035 |
| JECFA Number | 149 |
| BRN | 1740743 |
| Dielectric constant | 5.6(20℃) |
| Stability: | Stable. Flammable. Incompatible with strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 623-42-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Butanoic acid, methyl ester(623-42-7) |
| EPA Substance Registry System | Methyl butyrate (623-42-7) |
Safety information for Methyl butyrate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H225:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. |
Computed Descriptors for Methyl butyrate
Methyl butyrate manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 623-42-7 98%View Details
623-42-7 98%View Details
623-42-7 -
 Methyl butyrate CAS 623-42-7View Details
Methyl butyrate CAS 623-42-7View Details
623-42-7 -
 Methyl butyrate CAS 623-42-7View Details
Methyl butyrate CAS 623-42-7View Details
623-42-7 -
 Methyl butyrate CAS 623-42-7View Details
Methyl butyrate CAS 623-42-7View Details
623-42-7 -
![Methyl Butyrate [Standard Material for GC] CAS 623-42-7](https://img.chemicalbook.in//Content/image/CP5.jpg) Methyl Butyrate [Standard Material for GC] CAS 623-42-7View Details
Methyl Butyrate [Standard Material for GC] CAS 623-42-7View Details
623-42-7 -
 Methyl Butyrate CAS 623-42-7View Details
Methyl Butyrate CAS 623-42-7View Details
623-42-7 -
 Methyl butyrate CAS 623-42-7View Details
Methyl butyrate CAS 623-42-7View Details
623-42-7 -
 Methyl butyrate CAS 623-42-7View Details
Methyl butyrate CAS 623-42-7View Details
623-42-7