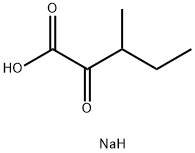
Sodium Butyrate
Synonym(s):Butyric acid sodium salt;Butyric Acid, Na, NaB, HDAC Inhibitor V;Sodium butyrate;Sodium Butyrate - CAS 156-54-7 - Calbiochem
- CAS NO.:156-54-7
- Empirical Formula: C4H7NaO2
- Molecular Weight: 110.09
- MDL number: MFCD00002816
- EINECS: 205-857-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
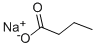
What is Sodium Butyrate?
Description
Sodium butyrate—more properly sodium butanoate—is a salt of butyric (butanoic) acid. If it comes into contact with even a trace of moisture, it emits a faint odor of its foul-smelling conjugate acid.
The butyrate ion, along with the analogous propionate ion, is purported to enhance gut health in humans and many other animal species. But you don’t have to take it as a supplement. Bacteria in the gut produce butyrate from dietary fiber, especially the fiber from legumes and nuts.
Two recent reports illustrate the benefits of butyrate. Yanfen Bai and Thomas J. Mansell* at Iowa State University (Ames) wrote, “The short-chain fatty acid [anion] butyrate plays critical roles in human gut health, affecting immunomodulation, cell differentiation, and apoptosis, while also serving as the preferred carbon source for colon cells.” To assist in studies of butyrate for therapeutic applications, the researchers developed a high-throughput biosensor that responds to intracellular concentrations.
In another account, Sanne Verhoog at the University of Bern (Switzerland) and collaborators there and at other institutions in Switzerland, Turkey, Germany, and the United States reviewed 29 studies about how dietary factors influence the populations of two beneficial gut bacteria, Akkermansia muciniphila (11 studies) and Faecalibacterium prausnitzii (25 studies). The studies involved a total of 1444 participants. Supplementation with several substances, including sodium butyrate, increased the abundance of A. muciniphila, whereas diets low in fermentable saccharides that produce butyrate lowered the quantity of the bacterium. (Butyrate was not considered in the case of F. prausnitzii.)
Chemical properties
Sodium butyrate is a white crystalline solid with an unpleasant smell that occurs in butter and animal fat as the glycerol ester. It is the sodium salt of butyric acid. It has various effects on cultured mammalian cells including inhibition of proliferation, induction of differentiation and induction or repression of gene expre.
The Uses of Sodium Butyrate
Sodium Butyrate is an inhibitor of histone deacetylase activity (HDAC) resulting in improvement of physiological disorders such as Duchenne muscular dystropy. In addition it is a possible antitumor agent when used in conjunction with other cytotoxic agents as they have been shown to treat carcinomas.
The Uses of Sodium Butyrate
Sodium butyrate has been used:
as a component in homogenization and immunoprecipitation buffer for HDAC inhibition in fly embryos and insect s2 cells.
as histone deacetylase inhibitor in breast cancer cell line MDA-MB-231, analysed by cell viability assay and viral replication assay.
to enhance the production of recombinant tissue-plasminogen activator (t-PA) in Chinese hamster ovary (CHO) cells in a bioreactor.
What are the applications of Application
Sodium Butyrate is an HDAC inhibitor, apoptosis inducer and can induce differentiation and gene expression and also prevent cell proliferation
Definition
ChEBI: Sodium butyrate is an organic sodium salt resulting from the replacement of the proton from the carboxy group of butyric acid by a sodium ion. It has a role as an EC 3.5.1.98 (histone deacetylase) inhibitor and a geroprotector. It contains a butyrate.
Benefits
Sodium butyrate, a butyrate, is used in supplements. The benefits of butyrate begin in the gut and extend to a host of other important body systems. It could fuel the gut lining, support healthy gut permeability. promote a healthy inflammation response in the gut, may fight colonic diseases and cancers, promote healthy blood sugar levels, supports normal proliferation of healthy yeast levels, promote cell differentiation, regulate the immune system , combat brain fog and improve sleep, and supports healthy blood pressure levels.
Biological Activity
Histone deacetylase inhibitor. Restores contextual memory in a transgenic mouse model of Alzheimer's disease. Shown to induce pancreatic progenitor formation. Directs the differentiation of mouse embryonic stem cells into hepatocytes when used in combination with the cytokine Activin A.
Biochem/physiol Actions
Sodium butyrate is a short-chain fatty acid that inhibits histone deacetylase (HDAC) in the millimolar range. Decreases Ca2+ release from intracellular stores. Induces apoptosis in several cell lines. Sodium butyrate induces the production of recombinant protein in Chinese hamster ovary (CHO) cells and other human cell lines. Sodium butyrate affects the proteome and gene expression pathways in the cells. It represses cell cycle related genes and modifies the genes involved in cell metabolism and apoptosis.
Storage
Room temperature
Purification Methods
Sodium butyrate [156-54-7] M 110.1. Prepare it by neutralising the acid with Na2CO3, and recrystallising it from EtOH. [Beilstein 2 IV 779.]
Properties of Sodium Butyrate
| Melting point: | 250-253 °C (lit.) |
| Density | 1.324 g/cm3 (30℃) |
| storage temp. | Store below +30°C. |
| solubility | Methanol (Slightly), Water (Slightly) |
| appearance | white crystals or powder |
| form | Amorphous Powder |
| color | White |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| BRN | 3629439 |
| Stability: | Stable. Incompatible with strong acids. |
| InChI | InChI=1S/C4H8O2.Na/c1-2-3-4(5)6;/h2-3H2,1H3,(H,5,6);/q;+1/p-1 |
| CAS DataBase Reference | 156-54-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium butyrate(156-54-7) |
| EPA Substance Registry System | Butanoic acid, sodium salt (156-54-7) |
Safety information for Sodium Butyrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium Butyrate
| InChIKey | MFBOGIVSZKQAPD-UHFFFAOYSA-M |
| SMILES | C(CC)C([O-])=O.[Na+] |
Sodium Butyrate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Sodium Butyrate 99%View Details
Sodium Butyrate 99%View Details -
 Sodium butyrate 99%View Details
Sodium butyrate 99%View Details -
 Sodium butyrate 156-54-7 99%View Details
Sodium butyrate 156-54-7 99%View Details
156-54-7 -
 156-54-7 Sodium butyrate 98%View Details
156-54-7 Sodium butyrate 98%View Details
156-54-7 -
 Sodium Butyrate CAS 156-54-7View Details
Sodium Butyrate CAS 156-54-7View Details
156-54-7 -
 Sodium butyrate, 98% CAS 156-54-7View Details
Sodium butyrate, 98% CAS 156-54-7View Details
156-54-7 -
 Sodium butyrate CAS 156-54-7View Details
Sodium butyrate CAS 156-54-7View Details
156-54-7 -
 Sodium Butyrate pure CAS 156-54-7View Details
Sodium Butyrate pure CAS 156-54-7View Details
156-54-7
