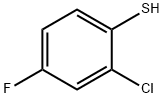
Methyl 2-[2-chloro-4-fluoro-5-[(3-oxo-5,6,7,8-tetrahydro-[1,3,4]thiadiazolo[3,4-a]pyridazin-1-ylidene)amino]phenyl]sulfanylacetate
Synonym(s):Methyl {2-chloro-4-fluoro-5-[(EZ)-5,6,7,8-tetrahydro-3-oxo-1H,3H-[1,3,4]thiadiazolo[3,4-a]pyridazin-1-ylideneamino]phenylthio}acetate
- CAS NO.:117337-19-6
- Empirical Formula: C15H15ClFN3O3S2
- Molecular Weight: 403.88
- MDL number: MFCD03792701
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
![Methyl 2-[2-chloro-4-fluoro-5-[(3-oxo-5,6,7,8-tetrahydro-[1,3,4]thiadiazolo[3,4-a]pyridazin-1-ylidene)amino]phenyl]sulfanylacetate Structural](https://img.chemicalbook.in/CAS/GIF/117337-19-6.gif)
What is Methyl 2-[2-chloro-4-fluoro-5-[(3-oxo-5,6,7,8-tetrahydro-[1,3,4]thiadiazolo[3,4-a]pyridazin-1-ylidene)amino]phenyl]sulfanylacetate?
The Uses of Methyl 2-[2-chloro-4-fluoro-5-[(3-oxo-5,6,7,8-tetrahydro-[1,3,4]thiadiazolo[3,4-a]pyridazin-1-ylidene)amino]phenyl]sulfanylacetate
Herbicide.
Definition
ChEBI: Fluthiacet-methyl is a methyl ester resulting from the formal condensation of the carboxy group of fluthiacet with methanol. A proherbicide for fluthiacet, it is used for the control of broad-leaved weeds in crops such as maize and soya. It has a role as an agrochemical, an EC 1.3.3.4 (protoporphyrinogen oxidase) inhibitor and a proherbicide. It is an organic sulfide, a methyl ester, a member of monochlorobenzenes, a member of monofluorobenzenes and a thiadiazolopyridazine. It is functionally related to a fluthiacet.
Metabolic pathway
Fluthiacet methyl is converted to its isomer, urazole, by
glutathione S-transferase (GST) and glutathione (GSH)
from some plants and rat liver microsomes. Fluthiacet
methyl inhibits Protox (protoporphyrinogen oxidase)
activity after conversion to the corresponding urazole by
GST and GSH. Fluthiacet methyl is chemically
converted to urazole with a thiol anion in media by the
nucleophilic reaction. It is also suggested that a free
acid of urazole, desulfated urazole, and the oxidative
ring-cleaved formyl degradation products result from
hydrolysis with esterase from soybean seedlings.
When fluthiacet methyl is administered orally to
rats in a single dose, 63-85% of the dose is excreted
in the feces and 10-23% in the urine, respectively,
after 48 h. The main metabolite is the isomer of
fluthiacet methyl (urazole) which is also identified in
the soil degradation products. Monohydroxylated
products on the pyridazine ring are identified as
metabolites by rats only.
Properties of Methyl 2-[2-chloro-4-fluoro-5-[(3-oxo-5,6,7,8-tetrahydro-[1,3,4]thiadiazolo[3,4-a]pyridazin-1-ylidene)amino]phenyl]sulfanylacetate
| Melting point: | 104.6° |
| Boiling point: | 503.5±60.0 °C(Predicted) |
| Density | 1.57±0.1 g/cm3(Predicted) |
| storage temp. | 0-6°C |
| pka | -2.86±0.20(Predicted) |
| form | neat |
| BRN | 11510131 |
| EPA Substance Registry System | Fluthiacet-methyl (117337-19-6) |
Safety information for Methyl 2-[2-chloro-4-fluoro-5-[(3-oxo-5,6,7,8-tetrahydro-[1,3,4]thiadiazolo[3,4-a]pyridazin-1-ylidene)amino]phenyl]sulfanylacetate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methyl 2-[2-chloro-4-fluoro-5-[(3-oxo-5,6,7,8-tetrahydro-[1,3,4]thiadiazolo[3,4-a]pyridazin-1-ylidene)amino]phenyl]sulfanylacetate
New Products
4-Piperidinemethanol Ethyl 2,4-Dihydroxy-6-methylnicotinate Ethyl isonicotinate 3-pyridine methanol N-Methyl 4-chloro-pyridine-2-carboxamide 2-Fluoro-6-iodobenzoic acid 2-((2,6-difluorobenzyl)(ethoxycarbonyl)amino)-4-((dimethylamino)methyl)-5-(4-nitrophenyl)thiophene-3-carboxylic acid Ethyl2-oxo-2,3,9,10-tetrahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(7H)-carboxylate Elinzanetant tert-butyl 2-(4-amino-6-chloropyrimidin-5-yloxy)ethylmethylcarbamate Phenylazomalononitrile 5,6 Dimethoxy-1-indanone 3-Iodophenylacetic acid 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole N Ethylmethylamine Ethyl Methanesulfonate N N' DimethylEthylenediamine Lead II Bromide Variamine Blue B Diazonium salt N N N'Trimethyl ethylenediamineRelated products of tetrahydrofuran

![Methyl 2-[2-chloro-4-fluoro-5-[(3-oxo-5,6,7,8-tetrahydro-[1,3,4]thiadiazolo[3,4-a]pyridazin-1-ylidene)amino]phenyl]sulfanylacetate](https://img.chemicalbook.in/CAS/GIF/117337-19-6.gif)
You may like
-
 Fluthiacet-methyl CAS 117337-19-6View Details
Fluthiacet-methyl CAS 117337-19-6View Details
117337-19-6 -
![4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%View Details
4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%View Details
3680-69-1 -
 4'-Benzyloxy-2-bromopropiophenone 98%View Details
4'-Benzyloxy-2-bromopropiophenone 98%View Details
35081-45-9 -
 53928-30-6 98%View Details
53928-30-6 98%View Details
53928-30-6 -
 5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 -
 4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
79307-93-0 -
 29676-71-9 99%View Details
29676-71-9 99%View Details
29676-71-9 -
 (R)-2-amino-N-benzyl-3-methoxypropanamide 98%View Details
(R)-2-amino-N-benzyl-3-methoxypropanamide 98%View Details
196601-69-1
