METHSUXIMIDE
Synonym(s):1,3-Dimethyl-3-phenyl-2,5-pyrrolidinedione
- CAS NO.:77-41-8
- Empirical Formula: C12H13NO2
- Molecular Weight: 203.24
- MDL number: MFCD00072132
- EINECS: 201-026-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
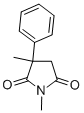
What is METHSUXIMIDE?
Toxicity
Acute overdoses may produce nausea, vomiting, and CNS depression including coma with respiratory depression. Levels greater than 40 μg/mL have caused toxicity and coma has been seen at levels of 150 μg/mL.
Description
Methsuximide is a succinimide that is converted to N-desmethylmethosuximide, a channel blocker that targets low threshold calcium currents. Methsuximide is a substrate of cytochrome P450 (CYP) isoform 2C19 that, in turn, inhibits CYP2C19-mediated metabolism of biguanides. Methsuximide has been shown to have anticonvulsant properties in clinical trials.
Chemical properties
Light Yellow Oil
Originator
Celontin,Parke Davis,US,1957
The Uses of METHSUXIMIDE
A calcium channel succinimide antiepileptic drug. Anticonvulsant.
Background
Mesuximide (or methsuximide) is an anticonvulsant medication. It is sold by Pfizer under the name Petinutin.
Indications
For the control of absence (petit mal) seizures that are refractory to other drugs.
What are the applications of Application
Methsuximide is a succinimide with anti-epileptic and anti-convulsant properties
Definition
ChEBI: Methsuximide is an organic molecular entity.
Manufacturing Process
100 g of α-phenyl-α-methylsuccinic acid and 110 g of 40% aqueous methyl amine are heated together at 200 to 250°C until no more distillate is obtained. Upon vacuum distillation of the residue, the N-methyl-α-phenyl-αmethylsuccinimide, of BP 121° to 122°C at 0.1 mm is obtained. After recrystallization from aqueous ethanol, this compound melts at 52° to 53°C.
brand name
Celontin (Parke-Davis).
Therapeutic Function
Anticonvulsant
Pharmacokinetics
Used in the treatment of epilepsy. Methsuximide suppresses the paroxysmal three cycle per second spike and wave activity associated with lapses of consciousness which is common in absence (petit mal) seizures. The frequency of epileptiform attacks is reduced, apparently by depression of the motor cortex and elevation of the threshold of the central nervous system to convulsive stimuli.
Clinical Use
Although methsuximide is less commonly used, it may be indicated for the control of absence seizures refractory to other drugs. Although it does not precipitate tonic-clonic convulsions, it often is combined with phenytoin or phenobarbital when absence seizures coexist with tonic-clonic symptoms. Much of the efficacy of methsuximide is attributed to its desmethyl metabolite. The half-life of methsuximide is between 2.6 and 4.0 hours, but the half-life for N-desmethylsuximide is 25 hours, causing it to accumulate substantially. Concentrations of greater than 40 g/mL may be associated with toxicity. Methsuximide is considered to be more toxic than ethosuximide.
Metabolism
Not Available
References
[1] nicholls, p. j., and orton, t.c. the physiological disposition of 14c-methsuximide in the rat. br.j.pharmacol. 45(1), 48-59 (1972).
[2] chen g, weston j k, bratton a c. anticonvulsant activity and toxicity of phensuximide, methsuximide and ethosuximide[j]. epilepsia, 1963, 4(1‐4): 66-76.
[3] sigler m, strassburg h m, boenigk h e. effective and safe but forgotten: methsuximide in intractable epilepsies in childhood[j]. seizure, 2001, 10(2): 120-124.
[4] wright j d, helsby n a, ward s a. the role of s‐mephenytoin hydroxylase (cyp2c19) in the metabolism of the antimalarial biguanides[j]. british journal of clinical pharmacology, 1995, 39(4): 441-444.
[5] guengerich f p. human cytochrome p450 enzymes[m]//cytochrome p450. springer us, 1995: 473-535.
Properties of METHSUXIMIDE
| Melting point: | 52-53° |
| Boiling point: | bp0.1 121-122° |
| Density | 1.1255 (rough estimate) |
| refractive index | 1.5440 (estimate) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | 2.8g/L(25 ºC) |
Safety information for METHSUXIMIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for METHSUXIMIDE
METHSUXIMIDE manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Methsuximide 98%View Details
Methsuximide 98%View Details -
 77-41-8 98%View Details
77-41-8 98%View Details
77-41-8 -
 Methsuximide CAS 77-41-8View Details
Methsuximide CAS 77-41-8View Details
77-41-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1