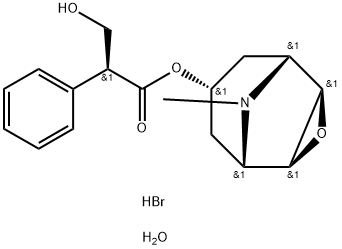
Methscopolamine bromide
Synonym(s):(−)-Scopolamine methyl bromide;Hyoscine methyl bromide;Methscopolamine bromide
- CAS NO.:155-41-9
- Empirical Formula: C18H24BrNO4
- Molecular Weight: 398.29
- MDL number: MFCD00078560
- EINECS: 205-844-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16

What is Methscopolamine bromide?
Absorption
Poorly and unreliably absorbed, total absorption is 10-25%.
Toxicity
Symptoms of a methscopolamine overdose include headache, nausea, vomiting, dry mouth, difficulty swallowing, blurred vision, dilated pupils, hot, dry skin, dizziness; drowsiness, confusion, anxiety, seizures, weak pulse, and an irregular heartbeat. In addition, a curare-like action may occur, i.e., neuromuscular blockade leading to muscular weakness and possible paralysis.
Chemical properties
Methscopolamine Bromide is an anticholinergic, which occurs as white crystals, or as a white odorless crystalline powder. It melts at about 225°C with decomposition. The drug is freely soluble in water, slightly soluble in alcohol, and insoluble in acetone and in chloroform.
Originator
Pamine,Upjohn,US,1953
The Uses of Methscopolamine bromide
METHSCOPOLAMINE BROMIDE is the methylated derivative of Scopolamine (S200000), a muscarinic antagonist that is similar to acetylcholine. METHSCOPOLAMINE BROMIDE have been used for the treatment of peptic ulcers by reducing acid secretion of the stomach and is used to treat morning sickness.
What are the applications of Application
(?)-Scopolamine methyl bromide is a competitive antimuscarinic compound
Indications
Used as adjunctive therapy for the treatment of peptic ulcer. Also used to treat nausea and vomiting due to motion sickness.
Background
A muscarinic antagonist used to study binding characteristics of muscarinic cholinergic receptors.
Definition
ChEBI: Scopolamine methyl bromide is a quaternary ammonium salt resulting from the reaction of the amino group of scopolamine with methyl bromide. It has a role as a muscarinic antagonist, an antiemetic, an antispasmodic drug and a parasympatholytic. It is a quaternary ammonium salt and a bromide salt. It is functionally related to a scopolamine.
What are the applications of Application
(-)-Scopolamine methyl bromide was administered to rats to inhibit the peripheral cholinergic effects induced by pilocarpine.
Manufacturing Process
In a one-liter separatory funnel, 94 g (0.215 mol) of scopolamine
hydrobromide trihydrate was dissolved in 250 ml of water, made alkaline by
shaking with 40 g (1 mol) of sodium hydroxide in 150 ml of water, and the
free base immediately extracted with ether. As scopolamine is somewhat
soluble in water, the aqueous layer was saturated with potassium carbonate
and again extracted with ether. The combined ether extracts were dried over
anhydrous magnesium sulfate and the ether removed by distillation, leaving
65 g (0.214 mol; 100% yield) of nearly colorless oil. Then 100 g (1.05 mols)
of cold methyl bromide was added to a chilled, 500-ml pressure flask
containing the 65 g of scopolamine, the flask stoppered tightly with a clamp,
and allowed to stand at room temperature for 96 hours.
The flask was cooled before opening, excess methyl bromide removed by
filtration, and the white solid washed thoroughly with dry ether. The yield of
crude scopolamine methyl bromide was 80g (94% yield; 93.5% over-all
yield).
The salt was recrystallized from 550 ml of alcohol; first crop, 70 g, MP 212° to
214°C; second crop, 6 g, MP 195° to 200°C. The combined crops were again
recrystallized from 500 ml of 3-A alcohol; MP 210° to 212°C. The third
recrystallization from 600 ml of alcohol yielded 64 g, MP 214° to 216°C, a
75% yield based on scopolamine hydrobromide trihydrate starting material.
Therapeutic Function
Spasmolytic
Biochem/physiol Actions
Competitive muscarinic acetylcholine receptor antagonist.
Pharmacokinetics
Methscopolamine is a muscarinic antagonist structurally similar to the neurotransmitter acetylcholine and acts by blocking the muscarinic acetylcholine receptors and is thus classified as an anticholinergic. Methscopolamine has many uses including the prevention of motion sickness. It is not clear how Methscopolamine prevents nausea and vomiting due to motion sickness. The vestibular part of the ear is very important for balance. When a person becomes disoriented due to motion, the vestibule sends a signal through nerves to the vomiting center in the brain, and vomiting occurs. Acetylcholine is a chemical that nerves use to transmit messages to each other. It is believe that Methscopolamine prevents communication between the nerves of the vestibule and the vomiting center in the brain by blocking the action of acetylcholine. Methscopolamine also may work directly on the vomiting center. Methscopolamine must be taken before the onset of motion sickness to be effective.
Metabolism
Little is known about the fate and excretion of methscopolamine.
Mode of action
methscopolamine bromide is used to treat gastrointestinal disorders such as peptic ulcers, irritable bowel syndrome, and stomach cramps. Its mechanism of action involves blocking the action of acetylcholine on smooth muscle cells in the digestive tract, thereby reducing spasms and increasing motility.
References
[1] ANDRé ROBERT JAMES E N. Effect of an anti-acetylcholine drug, methscopolamine bromide, on ulcer formation and gastric mucus[J]. Journal of Pharmacy and Pharmacology, 1964, 16 10: 690-695. DOI:10.1111/j.2042-7158.1964.tb07388.x.
[2] M A WASSERMAN R L G. Bronchospasmolytic effects of methscopolamine bromide.[J]. Journal of Pharmacology and Experimental Therapeutics, 1979, 211 1: 159-166.
[3] J A DANIEL. Methscopolamine bromide blocks hypothalamic-stimulated release of growth hormone in ewes.[J]. Journal of animal science, 1997, 75 5: 1359-1362. DOI:10.2527/1997.7551359x.
Properties of Methscopolamine bromide
| Melting point: | 214-217 °C (decomp) |
| refractive index | -24 ° (C=1, H2O) |
| storage temp. | Store at -20°C |
| solubility | H2O: 50 mg/mL |
| form | powder |
| color | white |
| optical activity | [α]25/D 25°, c = 5 in H2O(lit.) |
| Merck | 14,6003 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 155-41-9 |
| EPA Substance Registry System | 3-Oxa-9-azoniatricyclo[3.3.1.02,4]nonane, 7-[(2S)-3-hydroxy-1-oxo-2-phenylpropoxy]-9,9-dimethyl-, bromide (1:1), (1.alpha.,2.beta.,4.beta.,5.alpha.,7.beta.)- (155-41-9) |
Safety information for Methscopolamine bromide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Methscopolamine bromide
Methscopolamine bromide manufacturer
Prism Industries Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 155-41-9 Methscopolamine Bromide / Hyoscine Methylbromide 98%View Details
155-41-9 Methscopolamine Bromide / Hyoscine Methylbromide 98%View Details
155-41-9 -
 (-)-Scopolamine methyl bromide 155-41-9 99%View Details
(-)-Scopolamine methyl bromide 155-41-9 99%View Details
155-41-9 -
 Methscopolamine bromide 95% CAS 155-41-9View Details
Methscopolamine bromide 95% CAS 155-41-9View Details
155-41-9 -
 Scopolamine Methyl Bromide CAS 155-41-9View Details
Scopolamine Methyl Bromide CAS 155-41-9View Details
155-41-9 -
 Methscopolamine bromide CAS 155-41-9View Details
Methscopolamine bromide CAS 155-41-9View Details
155-41-9 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8