Methanesulfonyl chloride
Synonym(s):Mesyl chloride;Methanesulfonyl chloride;Methanesulfonyl chloride, Mesyl chloride;Methyl sulfonyl chloride
- CAS NO.:124-63-0
- Empirical Formula: CH3ClO2S
- Molecular Weight: 114.55
- MDL number: MFCD00007454
- EINECS: 204-706-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
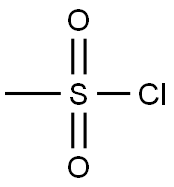
What is Methanesulfonyl chloride?
Chemical properties
Colorless to yellow liquid
Chemical properties
Pale yellow corrosive liquid. Unpleasant, pungent odor.
The Uses of Methanesulfonyl chloride
Methanesulfonyl chloride is used as a reagent for conversion of alcohols to mesylate esters such as methanesulfonate, which is an intermediate in substitution reactions, elimination reactions, reductions, and rearrangement reactions viz. Beckmann rearrangement. It is an electrophile and acts as a source of CH3SO2+ group. It is also used to prepare beta-chloro sulfones, methanesulfonamide and heterocyclic compounds containing five membered sultones.
The Uses of Methanesulfonyl chloride

To a solution of the SM (300 mg, 2.30 mmol) in DCM (6 mL) at 0 C was added TEA (0.64 mL, 4.6 mmol) followed by MeSO2Cl (0.21 mL, 2.76 mmol). The reaction mixture was stirred at 0 C for 30 min, then was allowed to warm to RT over 2 h. The mixture was quenched with H2O (10 mL) and extracted with DCM (2 x 20 mL). The combined organics were washed with H2O (20 mL), brine (20 mL), dried (Na2SO4), and concentrated. The resulting material was purified by silica gel column chromatography (35% EtOAc/hexane) to provide the product as a colorless gum. [310 mg, 64%]
The Uses of Methanesulfonyl chloride
In the synthesis of photographic and agricultural chemicals, pharmaceutical intermediates. As a stabilizer; catalyst; curing and chlorinating agent; precursor to methanesulfonic acid.
The Uses of Methanesulfonyl chloride
Methanesulfonyl chloride can be used for the mesylation of primary alcohols to synthesize the corresponding methanesulfonates. It may also be used for the conversion of amines to the corresponding sulfonamides.
General Description
A pale yellow corrosive liquid. More dense than water and insoluble in water. Very toxic by ingestion, inhalation, or skin absorption.
Reactivity Profile
Methanesulfonyl chloride reacts vigorously with water, steam, alkali, methylformamide. Emits toxic fumes of chloride and oxides of sulfur when heated to decomposition. A dangerous storage hazard. Reacts explosively with dimethyl sulfoxide [Buckley, A., J. Chem. Educ., 1965, 42, p. 674]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Potential Exposure
Chemical intermediate in various industries including pesticides, flame retardants, pharmaceuticals, plastics. A solvent, curing agent, and chemical stabilizer. Laboratory chemical.
Shipping
UN3246 Methanesulfonyl chloride, Hazard Class 6.1; Labels: 6.1-Poison Inhalation Hazard, 8-Corrosive material, Inhalation Hazard Zone B.
Purification Methods
Distil the sulfonyl chloride from P2O5 under vacuum. It is a strong IRRITANT.[Beilstein 4 IV 27.]
Incompatibilities
Vapors may form explosive mixture with air. Slowly reacts with water, releasing toxic and corrosive hydrogen chloride gas. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, DMSO, ethers, strong acids, strong bases.
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed
Properties of Methanesulfonyl chloride
| Melting point: | -33°C |
| Boiling point: | 60 °C/21 mmHg (lit.) |
| Density | 1.48 g/mL at 25 °C (lit.) |
| vapor pressure | 3.001-8.9hPa at 20-25℃ |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform, Hexanes |
| appearance | Colorless liquid |
| form | Liquid |
| color | Clear |
| Water Solubility | Miscible with alcohol, ether and organic solvents. Immiscible with water. |
| Sensitive | Moisture Sensitive |
| Merck | 14,5955 |
| BRN | 506297 |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 124-63-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Methanesulfonyl chloride(124-63-0) |
| EPA Substance Registry System | Methanesulfonyl chloride (124-63-0) |
Safety information for Methanesulfonyl chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H290:Corrosive to Metals H314:Skin corrosion/irritation H317:Sensitisation, Skin H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methanesulfonyl chloride
Methanesulfonyl chloride manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
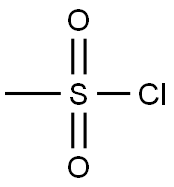

![2-[[(2-ethylphenyl)(2-hydroxyethyl)amino]methyl]-3,3-difluoro-Propanenitrile](https://img.chemicalbook.in/CAS/GIF/2647-14-5.gif)

You may like
-
 Methanesulfonyl chloride CAS 124-63-0View Details
Methanesulfonyl chloride CAS 124-63-0View Details
124-63-0 -
 Methane Sulphonyl Chloride CASView Details
Methane Sulphonyl Chloride CASView Details -
 Methane Sulfonyl Chloride CASView Details
Methane Sulfonyl Chloride CASView Details -
 Methanesulfonyl Chloride CASView Details
Methanesulfonyl Chloride CASView Details -
 Methanesulfonyl Chloride CASView Details
Methanesulfonyl Chloride CASView Details -
 Methane Sulfonyl Chloride CASView Details
Methane Sulfonyl Chloride CASView Details -
 Methane Sulphonyl ChlorideView Details
Methane Sulphonyl ChlorideView Details
124-63-0 -
 Methane Sulfonyl ChlorideView Details
Methane Sulfonyl ChlorideView Details
124-63-0
