Methacrylic anhydride
- CAS NO.:760-93-0
- Empirical Formula: C8H10O3
- Molecular Weight: 154.17
- MDL number: MFCD00008586
- EINECS: 212-084-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-12 18:21:52
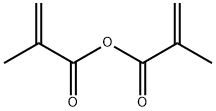
What is Methacrylic anhydride ?
Description
Methacrylic anhydride is a reactive monomer used primarily as a monomer in the preparation of polymers, particularly poly(methacrylic acid) and its copolymers. The reaction mechanism involves the formation of covalent bonds between methacrylic acid and acetic anhydride molecules, resulting in the formation of a novel compound. The newly formed compound then undergoes a series of reactions including hydrolysis and polymerisation. Methacrylic anhydride produces polymers with good chemical and thermal stability, which are used in a wide range of applications in various industries such as bio-renewable resins, pH-sensitive hydrogels, thermoset plastics, coatings, adhesives and plastics.
Chemical properties
Methacrylic anhydride is a colorless transparent liquid which reacts with water exothermically. It can also react with hydroxyl- and amino-groups present in some organic compounds leading to covalent attachment of methacryloyl moieties.
The Uses of Methacrylic anhydride
Methacrylic anhydride is a reactive monomer used for the preparation of different type of monomers under mild reaction conditions. It finds applications in light-curing coatings, cross-linked materials like synthetic resin. It acts as an intermediate for the preparation of fibers and resins.
What are the applications of Application
Methacrylic anhydride is a reactive monomer used primarily in preparation of other monomers under mild reaction conditions.
Preparation
To synthesize Methacrylic anhydride, sodium methacrylate (1 mol), propionyl chloride (3 mol), and a polymerization inhibitor (0.1 mol) were added to a reaction kettle. The mixture was heated to 90-100 ℃ and refluxed for 8 hours. Methacrylic anhydride was then obtained by distilling and collecting fractions at 100-105 ℃. The yield of the product was 98.33%.
What are the applications of Application
Methacrylic anhydride can be used as a starting material to synthesize:
Methacrylated chondroitin sulfate pH-sensitive hydrogels by copolymerization reaction. These hydrogels can be used in drug delivery systems.
High-performance lignin-based thermosets.
Gel polymer electrolyte, which is then integrated with the cathode to form high-performance lithium-ion batteries.
Methacrylic anhydride can be also used as a precursor to synthesize bio-renewable resins for composite applications. For example, it can be used to prepare soybean oil and a eugenol-based resin system. This resin possesses high thermal stability, and good mechanical properties, which makes it a suitable matrix for the pultrusion process.
General Description
Liquid.
Air & Water Reactions
Reacts exothermically with water to form methacrylic acid.
Reactivity Profile
Methacrylic anhydride reacts exothermically with water. The reactions are sometimes slow, but can become violent when local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases.
Fire Hazard
(Non-Specific -- Methacrylic Acid) Methacrylic anhydride may burn but may not ignite readily. Flammable/ poisonous gases may accumulate in tanks and hopper cars. Some of these materials may ignite combustibles (wood, paper, oil, etc.).
Synthesis
In a 300 ml four-necked flask equipped with a stirrer and a thermometer, pure air was charged with 148.6 mL of acetone, 49.5 g (0.46 mol) of potassium methacrylate, 49.5 mg of 4-methoxyphenol, and 49.5 mg of phenothiazine under an ice bath. It was stirred and dispersed. 25.0 g (0.22 mol) of MsCl was added dropwise thereto over 30 minutes. Then, the mixture was stirred under an ice bath, and when it was confirmed that the exotherm had subsided, the outside temperature was set to 25 ° C., and the reaction was followed by GC while aging for 1 hour. As a result, it was confirmed that MsCl had disappeared. The conversion rate of the crude body was 99.0%. After completion of the reaction, the mixture was filtered using a filter paper, washed with 100 mL of acetone three times, and then acetone was distilled off under reduced pressure to obtain 27.8 g of crude methacrylic anhydride (crude yield 101.0% with respect to MsCl, GC). Purity 98.0%) was obtained. Subsequently, the crude product was distilled under reduced pressure under pure air to obtain 26.1 g of methacrylic anhydride (b.p.53 ° C. (O.4 kPa), yield 94.8% with respect to MsCl, GC purity 99.6%).
Purification Methods
Distil the anhydride at 2mm pressure, immediately before use, in the presence of hydroquinone. [Beilstein 2 IV 1537.]
Properties of Methacrylic anhydride
| Melting point: | -20°C |
| Boiling point: | 87 °C13 mm Hg(lit.) |
| Density | 1.04 g/mL at 20 °C |
| vapor pressure | 90Pa at 20℃ |
| refractive index | n |
| Flash point: | 184 °F |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | Miscible with ethanol and ether. |
| form | clear liquid |
| color | Colorless to Light yellow to Light orange |
| Water Solubility | 98g/L at 20℃ |
| Sensitive | Moisture Sensitive |
| BRN | 1761982 |
| Stability: | Stable. Flammable. Moisture-sensitive. |
| CAS DataBase Reference | 760-93-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Methacrylic anhydride(760-93-0) |
| EPA Substance Registry System | Methacrylic anhydride (760-93-0) |
Safety information for Methacrylic anhydride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methacrylic anhydride
| InChIKey | DCUFMVPCXCSVNP-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
![Methacrylic Anhydride [stabilized with 1,1,3-Tris(3-tert-butyl-4-hydroxy-6-methylphenyl)butane] CAS 760-93-0](https://img.chemicalbook.in//Content/image/CP5.jpg) Methacrylic Anhydride [stabilized with 1,1,3-Tris(3-tert-butyl-4-hydroxy-6-methylphenyl)butane] CAS 760-93-0View Details
Methacrylic Anhydride [stabilized with 1,1,3-Tris(3-tert-butyl-4-hydroxy-6-methylphenyl)butane] CAS 760-93-0View Details
760-93-0 -
 Methacrylic anhydride, stabilized with ≈0.2% 2,4-dimethyl-6-tert-butylphenol CAS 760-93-0View Details
Methacrylic anhydride, stabilized with ≈0.2% 2,4-dimethyl-6-tert-butylphenol CAS 760-93-0View Details
760-93-0 -
 Methacrylic anhydride CAS 760-93-0View Details
Methacrylic anhydride CAS 760-93-0View Details
760-93-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
