Mercury(II) sulfide
Synonym(s):Mercuric sulfide red
- CAS NO.:1344-48-5
- Empirical Formula: HgS
- Molecular Weight: 232.65
- MDL number: MFCD00011046
- EINECS: 215-696-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
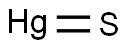
What is Mercury(II) sulfide?
Chemical properties
Fine, bright-scarlet powder.Insoluble in water and
alcohol.
Cinnabar, HgS, cherry red crystal to 5 mm among snow white rhombic dolomite, CaMg(CO3)2. Wanshan mine, Tongren Pref., Guizhou prov., China.
The Uses of Mercury(II) sulfide
For coloring plastics, sealing wax, and with FeSO4 for marking linen; manufacture of fancy colored papers; as pigment.
The Uses of Mercury(II) sulfide
Mercury(II) sulfide is used in paint, sealing wax and preservatives.
The Uses of Mercury(II) sulfide
Mercuric sulfide (HgS) is a fine, very brilliant scarlet powder that is deadly if ingested. Also known as the mercury ore cinnabar and metacinnabar, it is used as a pigment in the manufacture of paints.
Definition
cinnabar: A bright red mineralform of mercury(II) sulphide, HgS,crystallizing in the hexagonal system;the principal ore of mercury. Itis deposited in veins and impregnationsnear recent volcanic rocks andhot springs. The chief sources includeSpain, Italy, and Yugoslavia.
Air & Water Reactions
Readily oxidized as to be pyrophoric in air [Bretherick 1979. p. 120].
Reactivity Profile
Inorganic reducing agents, such as MERCURY(II) SULFIDE, react with oxidizing agents to generate heat and products that may be flammable, combustible, or otherwise reactive.
Hazard
Highly toxic by ingestion, inhalation, and skin absorption.
Health Hazard
Acute poisoning can result from inhaling dust concentrations of 1.2-8.5 mg/m 3 in air; symptoms include pain and tightness in chest, coughing, and difficulty in breathing. If ingested, toxicity depends on release of t he Hg ++ ion; chronic mercury poisoning can cause kidney, mental, and nervous disturbances. Dust irritates eyes and frequently causes allergic dermatitis; absorption through skin can cause systemic poisoning.
Properties of Mercury(II) sulfide
| Melting point: | 583.5 °C(lit.) |
| Boiling point: | 584°C |
| Density | 8.1 g/mL at 25 °C |
| solubility | insoluble in H2O; soluble in acid solutions, ethanol |
| form | Powder |
| color | red |
| Water Solubility | Soluble in water (0.00001 g/L). |
| Crystal Structure | Cubic, Sphalerite Structure - Space Group F(-4)3m |
| Merck | 14,5888 |
| Solubility Product Constant (Ksp) | pKsp: 52.4 |
| Exposure limits | ACGIH: TWA 0.025 mg/m3 (Skin) NIOSH: IDLH 10 mg/m3; TWA 0.05 mg/m3; Ceiling 0.1 mg/m3 |
| Stability: | Stable. |
| CAS DataBase Reference | 1344-48-5(CAS DataBase Reference) |
| EPA Substance Registry System | Mercury sulfide (HgS) (1344-48-5) |
Safety information for Mercury(II) sulfide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Mercury(II) sulfide
Mercury(II) sulfide manufacturer
Gurjar Chemicals Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 1344-48-5 Mercuric sulfide, red 99%View Details
1344-48-5 Mercuric sulfide, red 99%View Details
1344-48-5 -
 Mercury(II) sulfide CAS 1344-48-5View Details
Mercury(II) sulfide CAS 1344-48-5View Details
1344-48-5 -
 Mercury(II) sulfide red CAS 1344-48-5View Details
Mercury(II) sulfide red CAS 1344-48-5View Details
1344-48-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8