MERCURY(II) IODIDE
Synonym(s):Mercuric iodide red;Mercury diiodide;Mercury(II) iodide;Mercury(II) iodide red
- CAS NO.:7774-29-0
- Empirical Formula: HgI2
- Molecular Weight: 454.4
- MDL number: MFCD00011044
- EINECS: 231-873-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
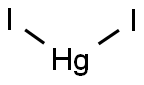
What is MERCURY(II) IODIDE?
Description
Mercuric iodide is a heavy, scarlet red, odorless, crystalline solid. It may be shipped as a red solution. It turns to a yellow powder at 127℃ and red upon cooling. Molecular weight= 454.40;Boiling point=(sublimes) 354℃; Freezing/Melting point=259℃. Hazard Identification (based on NFPA-704 M Rating System): Health 3, Flammability 0, Reactivity 0. Slightly soluble in water.
Chemical properties
Red Solid
Chemical properties
Mecuric iodide is a heavy, scarlet red, odorless, crystalline solid. It may be shipped as a red solution. It turns to a yellow powder @ 127℃ and red upon cooling
The Uses of MERCURY(II) IODIDE
Mercury(II) iodide is is used for preparation of Nessler's reagent, used for detection of presence of ammonia. It is a semiconductor material, used in some x-ray and gamma ray detection and imaging devices operating at room temperatures. In veterinary medicine, it is used in blister ointments in exostoses, bursal enlargement, etc.
The Uses of MERCURY(II) IODIDE
In animal chemistry for preparation of Nessler's Reagent (alkaline mercuric potassium iodide solution).
Definition
ChEBI: Mercury diiodide is a mercury coordination entity composed of mercury and iodine with formula HgI2.
General Description
A scarlet-red odorless tasteless powder. Sensitive to light. Insoluble in water and sinks in water. At elevated temperatures turns yellow but turns back to red upon cooling. Poison.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
MERCURY(II) IODIDE is a mild reducing agent. Reacts with sodium azide to form mercury(II) azide, which is shock, friction, and heat sensitive. Incompatible with acetylene, ammonia, chlorine dioxide, azides, chlorine trifluoride, calcium (because of amalgam formation), sodium carbide, lithium, rubidium, copper .
Hazard
Highly toxic by ingestion, inhalation, and skin absorption; strong irritant.
Health Hazard
All forms of exposure to MERCURY(II) IODIDE are hazardous. Acute systemic mercurialism may be fatal within a few minutes; death by uremic poisoning is usually delayed 5-12 days. Acute poisoning has resulted from inhaling dust concentrations of 1.2-8.5 mg/m 3 of air; symptoms include tightness and pain in chest, coughing, and difficulty in breathing. Ingestion causes necrosis, pain, vomiting, and severe purging. Contact with eyes causes ulceration of conjunctiv a and cornea. Contact with skin causes irritation and possible dermatitis; systemic poisoning can occur by absorption through skin.
Fire Hazard
Special Hazards of Combustion Products: Fumes from fire may contain toxic mercury vapor.
Safety Profile
A poison. When heated to decomposition it emits very toxic fumes of Hg and I-. See also MERCURY(I1) IODIDE.
Potential Exposure
Mercuric iodide is used in medicine and in analytical chemistry.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. Medical observation is recommended for 2448 h after breathing overexposure, as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a corticosteroid spray. Antidotes and special procedures for medical personnel: The drug NAP (n-acetyl penicillamine) has been used to treat mercury poisoning, with mixed success. Note to physician: For severe poisoning BAL [British AntiLewisite, dimercaprol, dithiopropanol (C3H8OS2)] has been used to treat toxic symptoms of certain heavy metals poisoning including mercury. Although BAL is reported to have a large margin of safety, caution must be exercised, because toxic effects may be caused by excessive dosage. Most can be prevented by premedication with 1-ephedrine sulfate (CAS: 134-72-5).
storage
Color Code—Blue: Health Hazard/Poison: Store in a secure poison location. Prior to working with this chemical you should be trained on its proper handling and storage. Mercuric iodide must be stored to avoid contact with chlorine trifluoride, sodium, and potassium, since violent reactions occur. Store in tightly closed containers in a cool, well-ventilated area away from light, acids, and heat. Protect containers from physical damage.
Shipping
This compound requires a shipping label of “POISONOUS/TOXIC MATERIALS” (solution). It falls in Hazard Class 6.1 and Packing Group II.
Purification Methods
Crystallise it from MeOH or EtOH and wash it repeatedly with distilled water (solubility is 0.006% at ~25o). It has also been mixed thoroughly with excess 0.001M iodine solution, filtered, washed with cold distilled water, rinsed with EtOH and Et2O, and dried in air. It changes colour reversibly to yellow at ~130o. [Friend Nature 109 341 1922.] POISONOUS.
Incompatibilities
Violent reaction with active metals; potassium, sodium, acids, chlorine trifluoride. Inorganic mercury compounds are incompatible with acetylene, ammonia, chlorine dioxide; azides, calcium (amalgam formation), sodium carbide, lithium, rubidium, copper. Mercury iodide is a mild reducing agent. Keep away from oxidizers. Reacts with sodium azide to form mercury(II) azide, which is shock-, friction-, and heat-sensitive. Incompatible with acetylene, ammonia, chlorine dioxide, azides, chlorine trifluoride, calcium (because of amalgam formation), sodium carbide, lithium, rubidium, copper (NIOSH, 1997)
Properties of MERCURY(II) IODIDE
| Melting point: | 259 °C(lit.) |
| Boiling point: | 354 °C(lit.) |
| Density | 6.36 |
| vapor pressure | 0.006 hPa (80 °C) |
| Flash point: | 350°C subl. |
| storage temp. | Store at RT. |
| solubility | potassium iodide solution: passes test |
| form | beads |
| Specific Gravity | 6.271 |
| color | White |
| Odor | Odorless |
| PH | 6-7 (50g/l, H2O, 20℃)(slurry) |
| Water Solubility | Insoluble inwater. Slightly soluble in alcohol, ether, acetone, chloroform, ethyl acetate, olive oil and castor oil. |
| Sensitive | Light Sensitive |
| Merck | 14,5879 |
| Solubility Product Constant (Ksp) | pKsp: 28.54 |
| Exposure limits | ACGIH: TWA 0.025 mg/m3; TWA 0.01 ppm (Skin) NIOSH: IDLH 10 mg/m3; TWA 0.05 mg/m3; Ceiling 0.1 mg/m3 |
| Stability: | Stable. Incompatible with strong oxidizing agents, potassium, sodium, interhalogens. Light-sensitive. |
| CAS DataBase Reference | 7774-29-0(CAS DataBase Reference) |
| EPA Substance Registry System | Mercuric iodide (7774-29-0) |
Safety information for MERCURY(II) IODIDE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for MERCURY(II) IODIDE
MERCURY(II) IODIDE manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
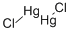
You may like
-
 Mercury(II) iodide, Assay-dried basis CAS 7774-29-0View Details
Mercury(II) iodide, Assay-dried basis CAS 7774-29-0View Details
7774-29-0 -
 Mercury(II) iodide, Assay-dried basis CAS 7774-29-0View Details
Mercury(II) iodide, Assay-dried basis CAS 7774-29-0View Details
7774-29-0 -
 Mercuric Iodide Red extrapure AR CAS 7774-29-0View Details
Mercuric Iodide Red extrapure AR CAS 7774-29-0View Details
7774-29-0 -
 Mercuric Iodide Red pure CAS 7774-29-0View Details
Mercuric Iodide Red pure CAS 7774-29-0View Details
7774-29-0 -
 Mercuric Iodide CAS 7774-29-0View Details
Mercuric Iodide CAS 7774-29-0View Details
7774-29-0 -
 Mercuric iodide, red, 99% CAS 7774-29-0View Details
Mercuric iodide, red, 99% CAS 7774-29-0View Details
7774-29-0 -
 Mercuric iodide red 99% AR CAS 7774-29-0View Details
Mercuric iodide red 99% AR CAS 7774-29-0View Details
7774-29-0 -
 Mercuric iodide red 99% CAS 7774-29-0View Details
Mercuric iodide red 99% CAS 7774-29-0View Details
7774-29-0