MERCUROUS CHLORIDE
Synonym(s):Calomel;Mercurous chloride
- CAS NO.:10112-91-1
- Empirical Formula: Cl2Hg2
- Molecular Weight: 472.09
- MDL number: MFCD00011043
- EINECS: 233-307-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
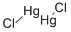
What is MERCUROUS CHLORIDE?
Description
Mercury(I) chloride, a colorless solid also known as the mineral calomel, is the compound with the formula Hg2Cl2, with
the connectivity Cl-Hg-Hg-Cl. It reacts with chlorine to form mercuric chloride, which resists further oxidation. Hg2Cl2 is
a linear molecule. Mercurous chloride forms through the reaction of elemental mercury and mercuric chloride.
Hg + HgCl2→Hg2Cl2
Chemical properties
White, rhombic crystals or crystalline powder; odorless. Stable in air but darkens on exposure to light.decomposed by alkalies. Insoluble in water, ether, alcohol, and cold dilute acids.
Chemical properties
Mercurous chloride forms tetrahedral white crystals, and, unlike the mercuric salt, is only very slightly soluble in water (about 2mg/L water at 20°C).
The Uses of MERCUROUS CHLORIDE
Dark green Bengal lights; calomel paper, mixed with gold in painting on porcelain; for calomel electrodes; as fungicide; in agriculture to control root maggots on cabbage and onions.
The Uses of MERCUROUS CHLORIDE
This compound is used by the pharmaceutical industry and is used also as a fungicide, as a poison, in fireworks, and to control maggots.
The Uses of MERCUROUS CHLORIDE
Calomel is used as a laboratory reagent, as a fungicide, and as a depolarizer in dry batteries.
Production Methods
Mercurous chloride is produced by exposing mercury metal to limited amounts of chlorine gas, insufficient to form mercuric chloride as the major product; it can also be prepared by precipitation from mercurous nitrate solution.
Definition
ChEBI: Dimercury dichloride is a mercury coordination entity.
General Description
Odorless white solid. Sinks in water.
Reactivity Profile
MERCUROUS CHLORIDE is incompatible with acetylene, ammonia, chlorine dioxide, azides, calcium (amalgam formation), sodium carbide, lithium, rubidium, copper .
Hazard
Toxic dose is uncertain.
Health Hazard
Acute poisoning can result from inhaling dust concentrations of 1.2-8.5 mg/m 3 in air; symptoms include pain and tightness in chest, coughing, and difficulty in breathing. Compound is an irritant, cathartic, or purgat ive; rarely, ``calomel sickness,'' a benign reaction with fever and rash, appears after about 1 week; seldom causes systemic poisoning but may be fatal if retained to 30-40 mg/kg. Contact with eyes causes mild irritation.
Carcinogenicity
An acute oral dose in humans of 1 g HgCl2 may cause corrosive damage to the GI tract; there is, however, little quantitative information on dose–effect relationships during low-level exposure to inorganic mercury. A dose of 2 g may be expected to increase mortality greatly among victims of the poison. Death from acute oral exposure is usually caused by cardiovascular collapse and renal failure. Ingestion of inorganic compounds may cause gastrointestinal corrosion and irritation, such as vomiting, bloody diarrhea, and stomach pains.
Environmental Fate
Calomel can generate reactive oxygen species and deplete glutathione levels. Both genotoxic and nongenotoxic mechanisms may contribute to renal carcinogenic effect of mercury.
Toxicity evaluation
Calomel decomposes gradually in the presence of sunlight. It slowly decomposes to mercury and mercuric chloride under aqueous conditions.
Properties of MERCUROUS CHLORIDE
| Melting point: | 400 °C (subl.)(lit.) |
| Boiling point: | 383°C |
| Density | 7.15 |
| vapor pressure | 1.7 mm Hg at 236 °C |
| storage temp. | Poison room |
| solubility | insoluble in ethanol, ethyl ether |
| form | powder |
| color | White |
| Specific Gravity | 7.15 |
| Odor | Odorless |
| Water Solubility | Soluble in (0.002g/L )water. |
| Sensitive | Moisture & Light Sensitive |
| Merck | 14,5894 |
| Solubility Product Constant (Ksp) | pKsp: 17.88(25°C) |
| Exposure limits | ACGIH: TWA 0.025 mg/m3 (Skin) NIOSH: IDLH 10 mg/m3; TWA 0.05 mg/m3; Ceiling 0.1 mg/m3 |
| Dielectric constant | 7.0(Ambient) |
| CAS DataBase Reference | 10112-91-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Mercurous chloride(10112-91-1) |
| EPA Substance Registry System | Mercurous chloride (10112-91-1) |
Safety information for MERCUROUS CHLORIDE
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. P405:Store locked up. |
Computed Descriptors for MERCUROUS CHLORIDE
| InChIKey | ZOMNIUBKTOKEHS-UHFFFAOYSA-L |
MERCUROUS CHLORIDE manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

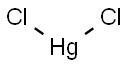

You may like
-
 Mercurous chloride, dihydrate, GR 99%+ CAS 10112-91-1View Details
Mercurous chloride, dihydrate, GR 99%+ CAS 10112-91-1View Details
10112-91-1 -
 Mercurous Chloride extrapure AR CAS 10112-91-1View Details
Mercurous Chloride extrapure AR CAS 10112-91-1View Details
10112-91-1 -
 Mercurous chloride, dihydrate CAS 10112-91-1View Details
Mercurous chloride, dihydrate CAS 10112-91-1View Details
10112-91-1 -
 Mercurous Chloride pure CAS 10112-91-1View Details
Mercurous Chloride pure CAS 10112-91-1View Details
10112-91-1 -
 Mercurous chloride 98% CAS 10112-91-1View Details
Mercurous chloride 98% CAS 10112-91-1View Details
10112-91-1 -
 Mercurous chloride 99.5% AR/ACS CAS 10112-91-1View Details
Mercurous chloride 99.5% AR/ACS CAS 10112-91-1View Details
10112-91-1 -
 Mercury(I) ChlorideView Details
Mercury(I) ChlorideView Details
10112-91-1 -
 Mercurous ChlorideView Details
Mercurous ChlorideView Details
10112-91-1
