MEPHENESIN
- CAS NO.:59-47-2
- Empirical Formula: C10H14O3
- Molecular Weight: 182.22
- MDL number: MFCD00004718
- EINECS: 200-427-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
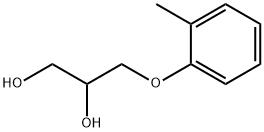
What is MEPHENESIN?
Toxicity
Mephenesin can produce hemolysis leading to hemoglobinuria with intravenous administration of concentrations greater than 10% . As a central nervous system depressant it can also produce paralysis and respiratory depression.
Originator
Tolserol,Squibb,US,1948
The Uses of MEPHENESIN
Mephenesin was used in the study of ligand-based virtual screening and design of antimalarial compounds.
The Uses of MEPHENESIN
muscle relaxant (skeletal)
Indications
Mephenesin was used for the treatment of muscle spasticity in diseases like Parkinson's or Multiple Sclerosis.
Background
Mephenesin is a synthetic cresol glyceryl ether which produces transient muscle relaxation and paralysis via central nervous system depression . It first entered use in the 1950s.
What are the applications of Application
Mephenesin is a chemical that relaxes muscle cells in vitro
Definition
ChEBI: 1-(2-methylphenyl)glycerol is a glycerol ether in which a single 2-methylphenyl group is attached at position 1 of glycerol via an ether linkage. It is an aromatic ether and a glycerol ether. It is functionally related to an o-cresol.
Manufacturing Process
Into an iron or copper reaction vessel having an efficient stirring device and furnished with a refluxing column and condenser, were charged 330 lb of high quality meta-cresol and 150 lb of glycerol, together with 25 lb of sodium acetate to serve as the catalyst in the reaction. The reaction mixture, of this composition, was then heated to 250°C. The water of the reaction distilled off during the heating as the ether formation proceeded, this removal of water from the reaction chamber being promoted by the presence of the excess of phenol, some of which also continued to distill over. Towards the end of the reaction, after about 12 hours, when about 60% of the glycerol had been converted, at which point the reaction slowed down and the distillate was mainly cresol, the batch was cooled and 50 gallons of water were added to it along with 150 lb of xylene. As the result of these additions and the cooling down of the material the batch stratified into an aqueous layer containing unreacted glycerol, polyglycerols and sodium acetate, and a nonaqueous layer containing the ethers that had been formed in the reaction, together with unreacted cresol which remained in the reaction chamber, dissolved in the xylene that had been added to the batch. The aqueous layer was then separated and the water content removed therefrom by evaporation to a degree suitable for the recovery of the glycerol and sodium acetate contents of the layer, for their reuse in the process in a succeeding batch therein. The separated nonaqueous layer containing the ethers was distilled to recover the xylene and cresol contents respectively as the early fractions of the layer thus subjected to distillation. The cresol thus recovered, together with the cresol recovered from the distillate obtained during the heating of the reaction mixture, was returned to the process for reuse in a succeeding batch. Redistillation of the ether mixture recovered is usually necessary and desirable, particularly from the point of view of removing last traces of cresol therefrom. The yield of mixed ethers in this example was about 200 lb, in the relative proportions stated of about 70 parts of monoether to 30 of diether.
brand name
Tolserol (Bristol-Myers Squibb);Bioglan m/q;Decontracyl-baum;Geno-sal;Glykresinum;Glyptol;Kencaps;Mefentil;Mepha-gesic;Mephesol;Midisalb-m;Myocalm;Myolisysin;Neo-xoline-m;Nochyrol;Relaxil-g;Rhex "hobein";Salimed compound;Walconesin.
Therapeutic Function
Muscle relaxant
World Health Organization (WHO)
Mephenesin, a centrally acting muscle relaxant and sedative, was introduced in 1948 and its use has subsequently been associated with some of the undesirable features of barbiturate use. It is of limited efficacy since it is shortacting and does not relieve the spasticity associated with chronic neurological disorders. It has therefore been largely superseded by benzodiazepines but it remains available in some countries.
Pharmacokinetics
Mephenesin reduced neuronal excitability leading to decreases action potentials to muscle fibers which ultimately produces a reduction in spasticity .
Metabolism
Not Available
Properties of MEPHENESIN
| Melting point: | 69-71 °C(lit.) |
| Boiling point: | 153-154 °C4 mm Hg(lit.) |
| Density | 1.0966 (rough estimate) |
| refractive index | 1.5320 (estimate) |
| Flash point: | 153-154°C/4mm |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in alcohol |
| form | Solid |
| pka | 13.52±0.20(Predicted) |
| color | White |
| Merck | 14,5850 |
Safety information for MEPHENESIN
| Signal word | Warning |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P321:Specific treatment (see … on this label). P405:Store locked up. |
Computed Descriptors for MEPHENESIN
MEPHENESIN manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 59-47-2 Mephenesin 99%View Details
59-47-2 Mephenesin 99%View Details
59-47-2 -
 MEPHENESIN 99%View Details
MEPHENESIN 99%View Details -
 Mephenesin 99%View Details
Mephenesin 99%View Details -
 Mephenesin 59-47-2 98%View Details
Mephenesin 59-47-2 98%View Details
59-47-2 -
 59-47-2 95-99%View Details
59-47-2 95-99%View Details
59-47-2 -
 Mephenesin 95% CAS 59-47-2View Details
Mephenesin 95% CAS 59-47-2View Details
59-47-2 -
 Mephenesin CAS 59-47-2View Details
Mephenesin CAS 59-47-2View Details
59-47-2 -
 3-(ORTHO-TOLYLOXY)-1,2-PROPANEDIOL CAS 59-47-2View Details
3-(ORTHO-TOLYLOXY)-1,2-PROPANEDIOL CAS 59-47-2View Details
59-47-2
