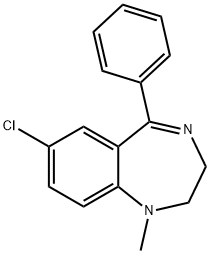
MEDAZEPAM
- CAS NO.:2898-12-6
- Empirical Formula: C16H15ClN2
- Molecular Weight: 270.76
- MDL number: MFCD00063407
- EINECS: 220-783-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:44:01
What is MEDAZEPAM?
Originator
Nobrium,Roche,Italy,1969
The Uses of MEDAZEPAM
Tranquilizer (minor).
Definition
ChEBI: Medazepam is an organic molecular entity.
Manufacturing Process
(A) Preparation of 4-Acetyl-7-Chloro-1,2,3,4-Tetrahydro-1-Methyl-5H-1,4- Benzodiazepin-5-one: A mixture of 68.5 g (0.37 mol) of 5-chloro-Nmethylanthranilic acid, 51 g (0.51 mol) of calcium carbonate, 76 g (0.37 mol) of bromoethylamine hydrobromide and 2.5 liters of water was stirred and heated under reflux for 3 hours. A solution of 23.4 g (0.26 mol) of anhydrous oxalic acid in 250 ml of water was slowly added to the refluxing mixture. The precipitated calcium oxalate was filtered off, and the filtrate adjusted to pH 7 with concentrated ammonium hydroxide. The filtrate was then concentrated to
dryness in vacuo and the residue heated on the steam bath with 400 ml of 6
N ethanolic hydrogen chloride until the residue was crystalline. Filtration gave
122 g of N-(aminoethyl)-5-chloro-N-methylanthranilic acid hydrochloride as a
solid.
A mixture of 100 g of this solid and 1 liter of acetic anhydride was stirred and
heated under reflux for 1.5 hours and then allowed to stand for 18 hours at
room temperature. The excess acetic anhydride was removed in vacuo, and
the residue was treated with one liter of water and ice and sufficient sodium
bicarbonate to make neutral. The solid was collected, sucked dry on the filter,
and triturated with hot ethanol. The ethanol solution on cooling gave 30.8 g of
4-acetyl-7-chloro-1,2,3,4-tetrahydro-1-methyl-5H-1,4-benzodiazepin-5-one.
(B) Preparation of 7-Chloro-1,2,3,4-Tetrahydro-1-Methyl-5H-1,4-
Benzodiazepin-5-one: A mixture of 25.25 g (0.1 mol) of 4-acetyl-7-chloro1,2,3,4-tetrahydro-1-methyl-5H-1,4-benzodiazepin-5-one, 33.3 ml (0.1 mol)
of 3 N sodium hydroxide and 350 ml of ethanol was heated under reflux for
15 minutes and then concentrated to dryness in vacuo. The residue was
treated with 500 ml of water, collected and washed with ethanol to give 20.2
g of 7-chloro-1,2,3,4-tetrahydro-1-methyl-5H-1,4-benzodiazepin-5-one.
(C) Preparation of 7-Chloro-2,3-Dihydro-1-Methyl-5-Phenyl-1H-1,4-
Benzodiazepine: A mixture of 4.7 g (22.6 mol) of 7-chloro-1,2,3,4-tetrahydro1-methyl-5H-1,4-benzodiazepin-5-one and 100 ml of phosphorus oxychloride
was heated in an oil bath at 100°C for 15 minutes. The solution was
concentrated to dryness in vacuo. The residue was partitioned between
methylene chloride and cold saturated sodium bicarbonate solution. The
methylene chloride phase was dried over sodium sulfate and sodium
bicarbonate, filtered, diluted with benzene and concentrated in vacuo to
produce crude 5,7-dichloro-2,3-dihydro-1-methyl-1H-1,4-benzodiazepine.
The residue was dissolved in 75 ml of tetrahydrofuran, treated with charcoal,
and sodium sulfate and filtered. This solution was added to a solution in 250
ml of tetrahydrofuran of phenyl magnesium bromide prepared from 17.7 ml
(0.17 mol) of bromobenzene. This mixture was stirred and heated under
reflux for 1 hour. It was then cooled and diluted with 400 ml of ether and
sufficient 3 N hydrochloric acid to make it acidic. The aqueous phase was
separated, adjusted to pH 8 with 3 N sodium hydroxide and extracted 3 times
with 200 ml of ether. The ether extracts were combined, washed with water
and dried over sodium sulfate. The residue left on removal of the ether in
vacuo was crystallized from petroleum ether to give 3.3 g of 7-chloro-2,3-
dihydro-1-methyl-5-phenyl-1H-1,4-benzodiazepine, according to US Patent
3,624,703.
A variety of alternative routes are outlined by Kleeman and Engel.
brand name
Nobrium (Hoffmann-LaRoche).
Therapeutic Function
Tranquilizer
Synthesis Reference(s)
The Journal of Organic Chemistry, 37, p. 3755, 1972 DOI: 10.1021/jo00797a001
Properties of MEDAZEPAM
| Melting point: | 95-97° |
| Boiling point: | 420.36°C (rough estimate) |
| Density | 1.1336 (rough estimate) |
| refractive index | 1.5749 (estimate) |
| storage temp. | -20°C |
| solubility | Methanol (Slightly), Water (Slightly) |
| pka | pKa 6.2(H2O) (Uncertain) |
| form | Solid |
| color | Yellow to Orange |
| Water Solubility | 10.83mg/L(37 ºC) |
Safety information for MEDAZEPAM
Computed Descriptors for MEDAZEPAM
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1