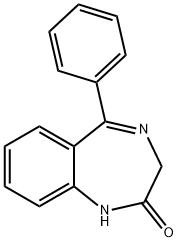
FLURAZEPAM
- CAS NO.:17617-23-1
- Empirical Formula: C21H23ClFN3O
- Molecular Weight: 387.88
- MDL number: MFCD00057324
- EINECS: 241-591-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
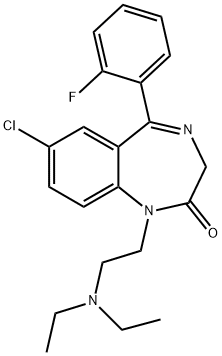
What is FLURAZEPAM?
Absorption
Flurazepam hydrochloride is rapidly (30 minutes) absorbed from the gastrointestinal tract
Toxicity
Coma, confusion, low blood pressure, sleepiness
Description
Flurazepam (CRM) (Item No. 18160) is a certified reference material that is structurally categorized as a benzodiazepine. It acts as a partial agonist of the benzodiazepine site on GABAA receptors, potentiating the action of GABA with an EC50 value of 930 nM in neuronal chick spinal cord cultures. Through this signaling mechanism, flurazepam produces anxiolytic and sedative properties that have been implemented clinically in the treatment of insomnia but also carry a potential for misuse. This product is intended for forensic and research purposes.
Chemical properties
Flurazepam is a pale yellow crystalline solid.
Originator
Dalmane, Roche,US,1970
The Uses of FLURAZEPAM
Anticonvulsant; relaxant (muscle); sedative-hypnotic Dalmane (Valeant.
Background
A benzodiazepine derivative used mainly as a hypnotic.
Indications
For short-term and intermittent use in patients with recurring insomnia and poor sleeping habits
Definition
ChEBI: Flurazepam is a 1,4-benzodiazepinone that is 1,3-dihydro-2H-1,4-benzodiazepin-2-one substituted by a 2-(diethylamino)ethyl group, 2-fluorophenyl group and chloro group at positions 1, 5 and 7, respectively. It is a partial agonist of GABAA receptors and used for the treatment of insomnia. It has a role as a sedative, an anticonvulsant, a GABAA receptor agonist and an anxiolytic drug. It is a 1,4-benzodiazepinone, an organochlorine compound, a member of monofluorobenzenes and a tertiary amino compound.
Manufacturing Process
13 grams of 5-(2-fluorophenyl)-7-chloro-2,3-dihydro-1H-1,4benzodiazepinone-(2) were dissolved in 100 ml of N,N-dimethylformamide andtreated with 10.3 ml of a solution of sodium methoxide in methanol containing 54 mmol or 2.95 grams of sodium methoxide. The resulting solution was stirred at about 20°C for 1 hour and then cooled in an ice-salt mixture to 0°C. A solution of diethylamino-ethyl chloride was prepared by dissolving 13.8 grams of diethylamino-ethyl chloride hydrochloride in cold dilute sodium hydroxide solution and extracting the base four times with 50 ml of toluene each time. The toluene extracts were combined, dried over anhydrous sodium sulfate, filtered and added to the reaction mixture.
The mixture was allowed to stand for 70 hours and then concentrated to a small volume under reduced pressure. The residue was dissolved in 100 ml of methylene chloride, washed with 75 ml of water, three times with 50 ml of saturated brine solution each time and filtered over neutral alumina (grade 1). The filtrate was evaporated to dryness and the resulting colorless oil taken up in ether, which was then saturated with hydrogen chloride. The pale yellow precipitate was filtered off and recrystallized from methanol/ether yielding 1[2-(diethylamino)ethyl]-5-(2-fluorophenyl)-7-chloro-2,3-dihydro-1H-1,4benzodiazepinone-(2) dihydrochloride as pale yellow rods melting at 190° to 220°C with decomposition, (from British Patent 1,040,548).
Therapeutic Function
Hypnotic
Pharmacokinetics
Flurazepam, a benzodiazepine derivative, is a hypnotic agent which does not appear to decrease dream time as measured by rapid eye movements (REM). Furthermore, it decreases sleep latency and number of awakenings for a consequent increase in total sleep time.
Pharmacokinetics
Chlorazepate is yet another benzodiazepine that is rapidly metabolized (3-decarboxylation) to N-desmethyldiazepam and so shares similar clinical and pharmacokinetic properties to chlordiazepoxide and diazepam.
Side Effects
The half-life of flurazepam is fairly long (~7 hours); consequently, it has the same potential as chlordiazepoxide and diazepam to produce cumulative clinical effects and side effects (e.g., excessive sedation) and residual pharmacological activity, even after discontinuation.
Safety Profile
Poison by intravenous routes. Moderately toxic by ingestion and subcutaneous routes. Experimental reproductive effects. Caution: May be habit forming. This is a controlled substance (depressant) listed in the US. Code of Federal Regulations, Title 21 Part 1308.14. When heated to decomposition it emits very toxic fumes of Cl-, Fand NOx. See also DIAZEPAM.
Potential Exposure
A Drug. Flurazepam is used as a seda tive in capsules or liquid form.
First aid
Eye Contact: Immediately remove any contactlenses and flush with large amounts of water for at least15 min, occasionally lifting upper and lower lids.Skin Contact: Remove contaminated clothing. Wash contaminated skin with water.
Metabolism
Flurazepam is administered orally as the dihydrochloride salt. It is rapidly 1N-dealkylated to give the 2′-fluoro derivative of N-desmethyldiazepam, and it subsequently follows the same metabolic pathways as chlordiazepoxide and diazepam.
Metabolism
Flurazepam is rapidly metabolized and is excreted primarily in the urine. Both hydroxyethyl flurazepam (the major metabolite) and N-desalkyl flurazepam are active. The N-desalkyl metabolite is slowly excreted in the urine as the conjugated form
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Store in tightly closed containers in a cool, well-ventilated area away from sources ofheat. If you are required to work in a “sterile” environmentyou require special training.
Shipping
UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Waste Disposal
It is inappropriate and possi bly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quanti ties of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
Properties of FLURAZEPAM
| Melting point: | 77-82° |
| Boiling point: | 551.4±50.0 °C(Predicted) |
| Density | 1.2089 (estimate) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: <6 mg/mL |
| form | solid |
| pka | pKa 1.90±0.05(H2O) (Uncertain);8.16±0.05 (Uncertain) |
| color | light yellow |
| EPA Substance Registry System | 2H-1,4-Benzodiazepin-2-one, 7-chloro-1-[2-(diethylamino)ethyl]-5-(2-fluorophenyl)-1,3-dihydro- (17617-23-1) |
Safety information for FLURAZEPAM
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P370+P378:In case of fire: Use … for extinction. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for FLURAZEPAM
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1